It’s an exciting time to be in the cornea field. Surgeons are working with thinner and thinner tissue, and in some cases, no tissue at all. With cell-injection therapies on the horizon, there’s a lot to look forward to. Here are some of the latest innovations in transplant techniques.
Simplifying Graft Unrolling
DMEK offers fast visual recovery, good final vision and a low rejection rate (1 percent, compared to 5 to 14 percent in DSAEK),1 but the procedure has a steep learning curve, owing mainly to the thin 10-µm grafts.
“There are multiple ways to inject and unscroll a DMEK graft,” says Marjan Farid, MD, a clinical professor of ophthalmology and director of the cornea, cataract and refractive surgery program and the ocular surface disease program at UC Irvine School of Medicine. “Surgeons should use whichever technique is most predictable and works best in their own hands.”
One method that aims to simplify graft delivery and soften DMEK’s learning curve is the pull-through technique.2 “Most corneal surgeons learn DSAEK first, so adapting the pull-through technique to DMEK reduces the technical difficulties associated with the procedure,” says Angeli Christy Yu, MD, a cornea fellow at Ospedali Privati Forlì in Forlì, Italy. “The pull-through technique involves bimanual delivery of the graft under low-flow irrigation, which allows for graft insertion in a controlled manner. Since the tissue’s natural tendency is to open endothelium-out, trifolding the graft endothelium-in facilitates the spontaneous unfolding of the graft within the anterior chamber, with minimal manipulation required.”
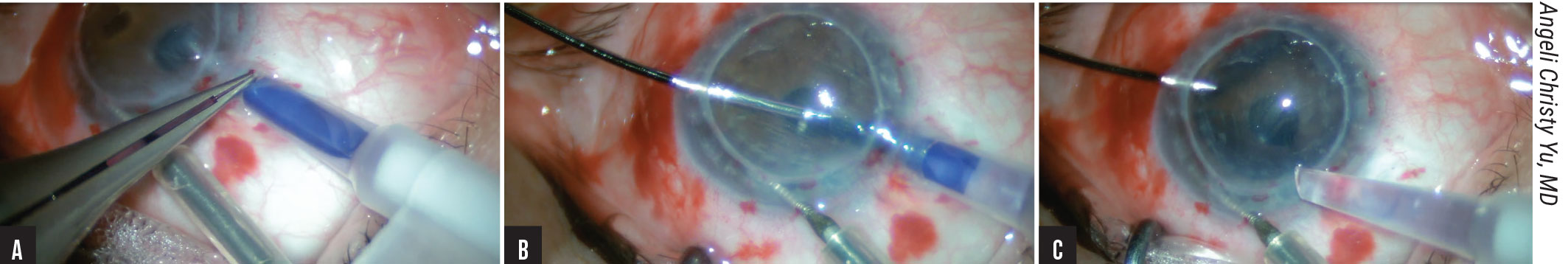 |
|
Figure 1. Trifolded DMEK graft insertion. (A) The IOL cartridge is inserted through a scleral tunnel incision. (B) Under continuous, low-flow irrigation, the edge of the DMEK graft is carefully grasped using dedicated anatomic forceps. (C) The trifolded endothelium-in DMEK graft is delivered bimanually into the AC and allowed to spontaneously unfold. |
The technique’s developer, Massimo Busin, MD, director of the corneal unit at Ospedali Privati Forlì and a professor of ophthalmology at the University of Ferrara, Italy, says that trifolded endothelium-in DMEK is suitable for complicated cases, but in most instances, such as in eyes with a poor surgical view, complex ocular anatomy or lower visual potential, he prefers ultra-thin DSAEK. “You can achieve excellent outcomes with either procedure,” he says. “However, DMEK is associated with faster visual rehabilitation. It also significantly reduces the risk of immunologic rejection due to the smaller amount of tissue, but it’s associated with higher rates of graft detachment requiring rebubbling.”
To perform the technique, Professor Busin first prepares a scleral tunnel and extends it into the clear cornea. After performing descemetorhexis under air, he creates an inferior peripheral iridotomy. The donor tissue is pre-marked, pre-stripped, stained with trypan blue and punched to 8.25 mm. He uses DMEK forceps (Moria, Antony, France) and trifolds the tissue, endothelium-in. Then he transfers the graft via a sterile soft contact lens (Sooft, Montegiorgio, Italy) and pulls it into the floor of the BSS-filled IOL cartridge funnel. He seals the cartridge with a silicone plug and rotates it to ensure the floor becomes the ceiling of the cartridge funnel as it’s inserted through the scleral tunnel incision (Figure 1A). Under low-flow continuous irrigation through a dedicated anterior chamber maintainer (Moria SA), he grasps the edge of the DMEK graft (Figure 1B) and bimanually delivers it into the AC, allowing for spontaneous unfolding (Figure 1C). He then injects air to attach the graft to the recipient cornea and inserts a 30-gauge needle obliquely to inject additional air to achieve complete air-fill. “I close the conjunctiva with cauterization,” he says. “The incisions can be left sutureless.”
Thomas John, MD, a clinical associate professor at Loyola University Chicago, says that time and staining may be a factor in graft unrolling. “We found that staining the donor Descemet’s membrane graft with trypan blue (Vision Blue 0.06%, DORC) may decrease DM elasticity and increase its stiffness,”3 he says. “Initially, the stained graft is often difficult to unroll, but if you wait a bit after staining, unrolling usually becomes easier.”
One study reported that grafts can be safely and effectively stained with 0.06% trypan blue for up to five minutes, but longer staining times with higher concentrations, such as 0.15%, resulted in decreased endothelial cell density approaching statistical significance.4
Dr. John adds that using a ball-tipped instrument on the outer corneal surface can also aid graft unrolling. He designed his own. “Applying the ball-tip on the patient’s outer cornea displaces fluid within the AC. The fluid currents you create by depressing the cornea help in unfolding the DM,” he says.
A Technique for DMEK in Complex Eyes
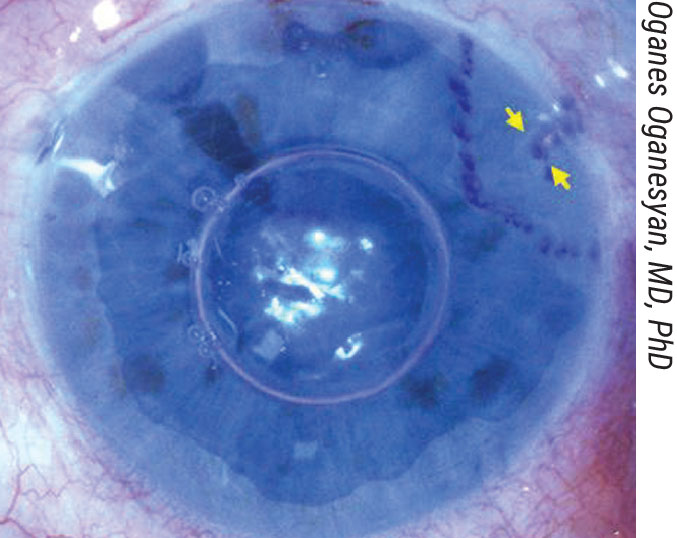
|
|
Figure 2. The three-quarter DMEK mega graft (11 to 12 mm) is placed centrally, with the missing quarter positioned at the tube shunt location. This graft shape is thought to reduce endothelial cell loss by avoiding direct contact with the drainage device. |
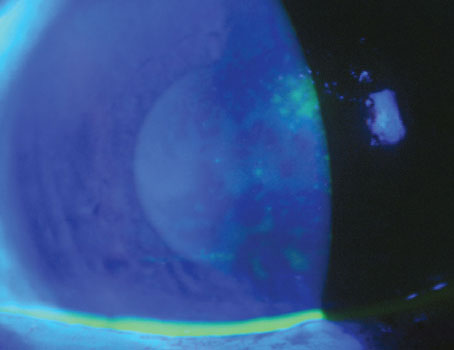
Many surgical modifications for DMEK are emerging to address complicated eyes, such as those with glaucoma drainage devices (GDD). Failure rates in these eyes after DMEK and DSEK at 24 months are high, and experts believe this may be due to mechanical damage to the donor tissue caused by intermittent or constant touch between the tube shunt and graft.
One new technique called three-quarter DMEK was described in a case series published last year by Gerrit Melles, MD, PhD, of the Netherlands Institute for Innovative Ocular Surgery, and colleagues.5 It’s intended for eyes with pseudophakic bullous keratopathy and GDD. The approach uses a ‘mega graft,’ a large-diameter graft modified into a ¾ shape. “We developed this technique to better facilitate graft positioning onto the recipient posterior stroma, while optimizing the spatial separation between the graft and silicone tube,” explains Oganes Oganesyan, MD, PhD, of the Helmholtz Moscow Institute of Eye Diseases. “Using a mega graft (11 to 12 mm) may compensate for the missing tissue quarter, in terms of absolute cell numbers, especially since large-diameter grafts include the corneal periphery, where ECD is supposedly higher.”
When harvesting the graft, the trabecular meshwork remains intact and is pulled together with the mega graft. Dr. Oganesyan positions the donor tissue stromal surface-down on a soft contact lens and dissects two triangle-shaped portions to remove a quarter of tissue. During the procedure, he creates an 11- to 12-mm descemetorhexis, except in the area at least 1.5 to 2 mm to each side of the shunt and its tip. In Dr. Oganesyan’s technique, the graft is stained with typan blue and aspirated into an 18-gauge intravenous catheter attached to a 2-ml syringe filled with BSS. After injecting the graft into the AC, he uses indirect manipulation from the outer cornea to position the graft centrally, endothelium-side down, and partially unrolls it to achieve visualization of the site with the quarter missing. “We use gentle taps with a cannula to rotate the graft into the proper position (Figure 2). After it’s completely unfolded, we pressurize the AC with a 100-percent air fill, which is left unreleased.”
A Tool for Real-time Visualization Intraoperative-OCT is an emerging technology that enables surgeons to visualize the cornea and anterior segment landscape in real time during surgery. “When you need higher optical resolution or your visual identification of tissue is compromised—say the view through the recipient cornea is limited due to corneal edema or some intraoperative bleeding—then i-OCT is very helpful,” says Thomas John, MD, of Chicago. “It’s also useful for titrating your air bubbles to achieve a more uniform attachment and visualization of the DM attachment contour to the inner surface of the recipient cornea. If you have i-OCT, it’s better to use it than to find gaps between the donor and recipient cornea at the slit lamp the next day and take the patient back to the OR to inject more air and optimize the donor-recipient interface.” Marjan Farid, MD, of UC Irvine School of Medicine, says i-OCT is especially useful for surgeons doing lamellar corneal transplantation, whether it’s an EK or DALK procedure. “To be able to see the layers of the cornea and ensure the tissue orientation is correct or that there’s no interface fluid or irregularity is a real advantage,” she says. “Hopefully we’ll see it integrated more and more as it develops." —CL |
In a case series of Dr. Oganesyan’s technique, no intraoperative or postop complications were noted.5 Average central ECD was 1,093 ±74 cells/mm2 at 12 months postop, demonstrating a decrease of 58 percent from preop values. BCVA increased from count-fingers to 20/60 at 12 months, and three-quarter DMEK remained stable with clear corneas up to 24 months.
Another DMEK technique for complex eyes was described by Professor Donald Tan, MBBS, FRCOphth(UK), a founder of the Singapore National Eye Center. His hybrid DMEK technique is intended for eyes with tube shunts, trabeculectomy, aphakia, aniridia and previous vitrectomy or keratoplasty.6 H-DMEK involves a bimanual pull-through technique using DSAEK-prepared donor stroma as a carrier, the EndoGlide Ultrathin DSAEK donor insertion device and an endothelium-in configuration. The case series he described in 2019 included 85 eyes with bullous keratopathy, FECD or failed grafts.6 H-DMEK was performed in 37 complex eyes. Four required rebubbling for graft detachment. Graft failure occurred in two cases. Overall endothelial cell loss was 32.2 ±20.5 percent at six months.
Ultra-thin DSAEK
Ultra-thin DSAEK is a recent development that uses grafts thinner than 100 µm to provide improved visual results. “The procedure is potentially more predictable than DMEK, with a lower risk of misfolding and rebubbling (15- vs. 3-percent rate),” says Rita Mencucci, MD, a professor at the School of Ophthalmology at the University of Florence, Italy. “We might call it ‘ultimate DSAEK’ because it implies the benefits of a ‘simple’ DSAEK with results comparable to DMEK.”
She and her colleague Michela Cennamo, MD, point out that UT-DSAEK’s requirements are similar to those of DMEK. A normal anterior segment without iris abnormalities, previous tube shunt surgery, IOL issues, an unstable AC or corneal issues is ideal.
“There’s a magic formula for UT-DSAEK, and that’s to keep it simple,” says Dr. Mencucci. “I use very basic DSAEK instrumentation—an inverted Price-Sinskey hook to perform descemetorhexis, a scraper to remove the endothelium and a Busin glide with a pull-through technique to insert the graft. In UT-DSAEK, you don’t have to change surgical maneuvers, in comparison to DSAEK.”
Dr. Mencucci says carefully controlling the turbulence of the fluids in the AC will help avoid loss of correct graft orientation. “The crucial moment for UT-DSAEK is right after graft injection,” she says. “Be careful that the graft doesn’t flip. It’s difficult to find the correct orientation of an upside-down UT-DSAEK graft.”
The literature regarding visual outcomes in UT-DSAEK compared to DMEK is controversial. “Some studies report better visual outcomes after DMEK and other studies have found similar BCVA between the two,” says Dr. Cennamo. “In our study comparing outcomes in fellow eyes, we found similar BCVA at 12 months postop.7 Moreover, we found that posterior corneal aberrations were significantly lower after DMEK than UT-DSAEK, while anterior aberrations didn’t differ significantly.”
“With regard to postop endothelial cell count, ECD loss rate was higher after DMEK,” Dr. Mencucci says. “This is probably caused by increased handling of DMEK tissue during surgery. However, ECD wasn’t significantly different between the two groups. DMEK outperforms UT-DSAEK in contrast sensitivity, especially in mesopic conditions and at intermediate spatial frequencies. For its quicker postop recovery and similar or better VA and lower rejection rates, I prefer DMEK even though its adoption is still limited in complicated cases.”
A Novel Corneal Layer
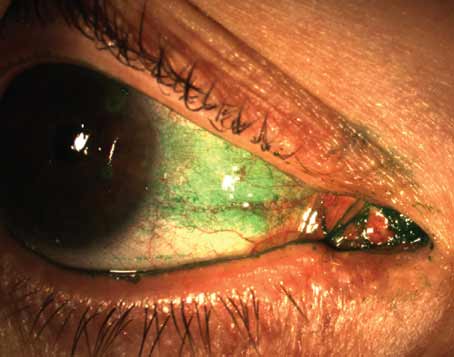
Pre-Descemet’s EK is a new technique pioneered by Harminder Dua, MD, FRCS, of Queens Medical Centre in Nottingham, U.K.8 It’s based on his team’s discovery of a novel corneal layer: pre-Descemet’s layer. In PDEK, the surgeon harvests a donor graft from PDL by creating a type-1 bubble, staining the inside of the bubble with trypan blue to mark the graft edges and then cutting around it to harvest the graft.
Any patient requiring EK for a corneal disorder is well-suited for PDEK, including eyes with pseudophakic bullous keratopathy with dense corneal scarring at the mid-stroma level (Figure 3), says Priya Narang, MS, director of the Narang Eye Care & Laser Centre in Ahmedabad, India. “There are no clear-cut contraindications for PDEK, but failure to create a type-1 bubble in the donor graft could be considered one clinical situation in which PDEK can’t be performed,” she notes. “If pre-cut PDEK donor grafts are available, there’s no issue of donor tissue loss.
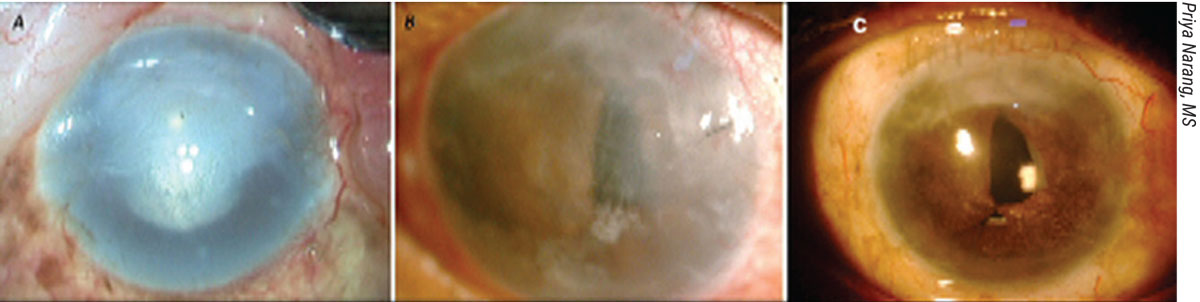 |
|
Figure 3. Pre-Descemet’s endothelial keratoplasty (PDEK). (A) Preop image of pseudophakic bullous keratopathy with corneal scarring. (B) One month after IOL explantation and glued IOL fixation, followed by PDEK and pupilloplasty. (C) Four months postop, with 20/40 vision. |
“One of the major advantages with PDEK is that it allows for the use of infant and young donor tissue,” she adds. “This indirectly allows us to use a graft that has a relatively higher endothelial cell count, compared to grafts from older donors used in DMEK procedures. PDEK also obviates the need for microkeratomes in obtaining donor lenticules, making it a pocket-friendly and surgeon-dependent technique. Another advantage is the additional splinting effect of the PDEK graft, due to the presence of PDL. This facilitates easier handling of the graft with a more controlled, easier unrolling in the AC.” A more detailed description of this technique can be found in Review’s June 2020 issue, or online at bit.ly/Cornea_00.
Descemet’s Stripping Only
While there will always be a place for keratoplasty procedures, more methods that involve less tissue, or even no tissue at all—like Descemet’s Stripping Only—are emerging. Kathryn Colby, MD, PhD, the Elisabeth J. Cohen Professor and chair of the Department of Ophthalmology at NYU Grossman School of Medicine, performed her first DSO case in January 2014 after seeing a growing body of evidence for corneal endothelial regeneration and spontaneous clearance in Fuchs’.9-11 In 2016, she published a case series of 11 patients (13 eyes) treated with her new technique.
DSO is indicated for mild to moderate FECD, but Dr. Colby says she’s heard case reports of the technique being used in other types of focal corneal edema, such as herpetic corneal edema. “It won’t work for pseudophakic bullous keratopathy because the patient must have an adequate amount of peripheral endothelium,” she notes. “The best candidates will have central guttae affecting 4 to 5 mm of the central cornea and an unaffected peripheral endothelium. If a patient has guttae all the way out to the periphery, I don’t offer DSO but instead do an EK.”
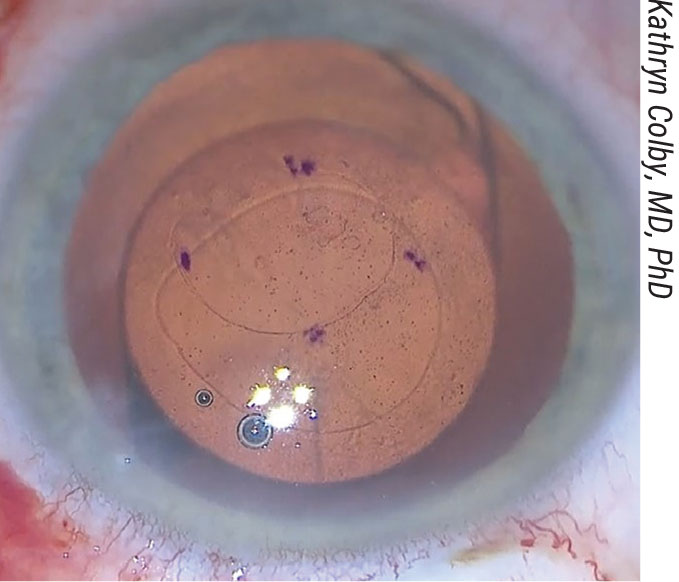
|
|
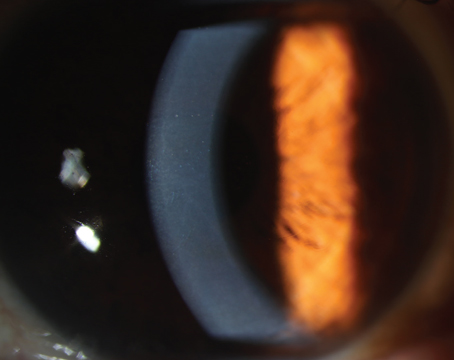
Figure 4. Kathryn Colby, MD, PhD, marks on the anterior cornea the amount she plans to remove in Descemet’s Stripping Only. |
There are several ways to remove the endothelium. Dr. Colby begins with a 2- to 3-mm clear or near-clear corneal incision, then marks the amount she plans to remove on the anterior surface of the cornea (Figure 4). “Right now, I use the Gorovoy forceps [Moria],” she says. “The blunt tip makes it very easy to do a controlled tear of the appropriate size. We know now that a smooth-edge tear helps endothelial cell migration.”
Other options include a reverse Sinskey hook, MST forceps or the I/A handpiece, she says. “I used the reverse Sinskey before the Gorovoy forceps were available. Some surgeons use the I/A handpiece to pull the endothelium off once they make a break through the endothelium and create an edge.”
The postoperative healing time for DSO is slightly longer than for DMEK or DSAEK, at about three to six weeks, but Dr. Colby says this downside is well-compensated for by the procedure’s benefits. “Every patient is swollen afterwards and their vision is blurry until the endothelium heals, but there’s no risk of rejection and no need for chronic steroids with DSO,” she explains. “It’s also a cost-effective surgery because there’s no expensive corneal tissue involved. Deepinder K. Dhaliwal, MD, of the University of Pittsburgh Eye Center, published a study comparing DMEK and DSO in 2018. She reported that both procedures resulted in good vision, and that DSO’s healing time was longer, but DMEK had more complications, namely rebubbling. There’s no rebubbling after DSO because there’s no tissue.”
Dr. Colby says the more recently published DSO case series are seeing much higher clearance rates than earlier cases, with the use of smooth-edge tears and ROCK inhibitors.
Help from ROCK Inhibitors
ROCK inhibitors stand to play an important role in endothelial procedures. “Greg Maloney, MBBS, MMed, FRCSC, of the Sydney Eye Hospital in Australia, rescued a couple of slow-to-clear DSO patients in 2016 with the ROCK inhibitor ripasudil [Glanatec, Kowa],” Dr. Colby says. “Since then, I’ve been telling my DSO patients about ROCK inhibitors. I can’t prescribe ripasudil because it’s not approved in the U.S., but if my patients choose to get it online, I can follow them while they’re using it. Most tend to choose it; they use it four times daily.”
Ripasudil is a 0.4% hydrochloride hydrate that’s been approved for use in glaucoma and ocular hypertension in Japan since 2014. Netarsudil (Rhopressa, Aerie), another ROCK inhibitor, is an amino-isoquinolone amide that received FDA approval in December 2017 but isn’t available yet.
“We have a multicenter, multinational trial (K-321: NCT04250207) going on now for ripasudil, sponsored by the manufacturer Kowa,” adds Dr. Colby, who serves as the U.S. chair for the trial through a research contract with NYU. “We recently completed enrollment and anticipate some topline results by the end of the year. Assuming they’re positive, we’ll go forward with a Phase III trial next year.”
When applied to cultured human corneal endothelial cells, ROCK inhibitors modulate their adhesion to the substrate, says Naoki Okumura, MD, PhD, of the department of biomedical engineering at Doshisha University, Kyoto City, Japan. “Human CECs recovered from a culture plate undergo dissociation-induced Rho/ROCK/MLC signaling activation, which results in impairment of cell adhesion,” he explains. “The ROCK inhibitor enhances cell adhesion by counteracting the dissociation-induced activation of this signaling pathway.”
Cell Injection Therapy
Cell injection is one of the developing cell-based approaches to corneal endothelial disorders that makes use of in vitro cultured hCECs. In place of a donor tissue transplant, these cultured cells are injected into the eye with the goal of adhering to and clearing the cornea.
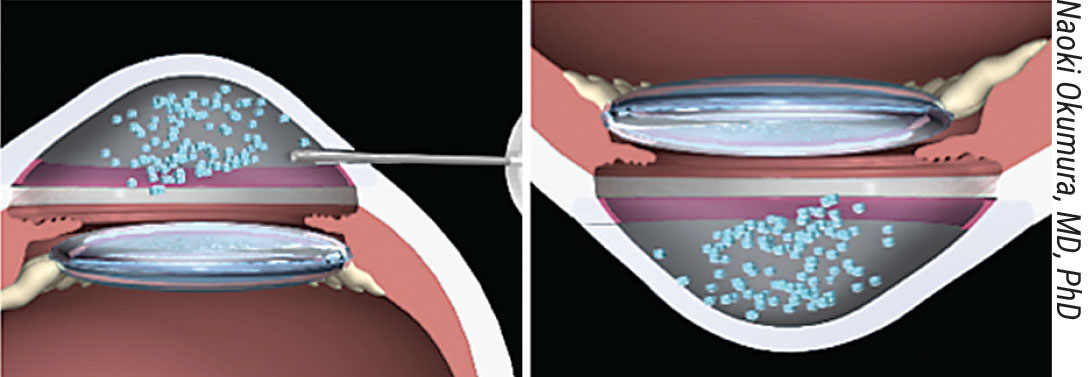
|
|
Figure 5. After cell injection, the patient lies face down to encourage the cells to adhere to the stroma. |
“Cell injection has multiple benefits,” says Dr. Farid. “With one donor cornea, you can potentially treat thousands of diseased corneas. In a worldwide shortage of donor corneas, this is a huge step and an advance in terms of making tissue available to patients who wouldn’t have access in the past.
“Cell injection will also simplify the procedure,” she says. “Instead of a full EK procedure, we could inject cells into the anterior chamber and position the patient face-down for a few hours while the cells adhere and start to clear the cornea.”
Dr. Okumura is currently developing pharmaceutical and tissue-engineering treatments for corneal endothelial dysfunction, including cell injection therapy. He says that in addition to enhancing cell adhesion, the ROCK inhibitor Y-27632 demonstrates other effects on cultured hCECs, including promotion of proliferation and suppression of apoptosis.
“In 2013, we initiated a first-in-human clinical trial of cell-based therapy at the Kyoto Prefectural University of Medicine,” he says. “By 2017, thirty-five patients with corneal endothelial decompensation had undergone cultured hCEC-injection therapy. We reported the clinical results of the first 11 cases in 2018.12 All 11 eyes recovered corneal transparency. After two years, all patients exhibited improved visual acuity, with VA better than 0.8 decimal (20/25 Snellen) in nine of 11 patients.”
He says that any cells for clinical use should be cultured in a cell processing center to ensure safety and efficacy. “Some companies will probably develop cultured hCEC products,” he says. “Then they’ll ship them to the hospital as ready-to-use products.”
Eye Banks and the Tissue Issue Eye banks and the pre-prepared tissue they offer have become indispensable to many corneal surgeons. “I consider eye-bank-prepared tissue superior to surgeon-prepared tissue,” says Marjan Farid, MD, of UC Irvine School of Medicine. “Surgeons don’t prepare tissue day in and day out, and they can potentially introduce more errors into the process. Tissue prepared by an eye-bank technician decreases surgeon variability and improves predictability and surgical time. Now eye banks are pre-loading DMEK and DSAEK tissue into cannulas like the Geuder cannula or the Endoserter, which makes the surgery more straightforward,” she says. “It saves time injecting the tissue into the AC and unscrolling it.” Eye banks do important work, but in many countries, they’re limited by inadequate supply. It’s estimated that globally, there’s only one corneal graft available for every 70 eyes.15 Descemet’s Stripping Only and cell injection therapies that require no donor tissue or can repurpose a single donor to treat multiple patients may be what’s needed to address the shortage, but those therapies aren’t suitable for every case. In cases where a transplant is required, researchers are investigating using smaller grafts. Quarter DMEK, described last year by Gerrit Melles, MD, PhD, and his colleagues, has the potential to quadruple endothelial graft availability, according to researchers in the Netherlands.16 They reported positive one- and two-year clinical outcomes, where all 19 eyes reached a BCVA of ≥20/40 at six months, 95 percent of eyes (n=18) reached ≥20/25 and 42 percent of eyes (n=9) reached ≥20/20. BCVA remained stable at two years. Mean donor ECD decreased from 2,842 ±139 cells/mm2 before implantation to 913 ±434 cells/mm2 at six months, representing a 68-percent decrease. ECD decreased by 70 and 74 percent at 12 and 24 months, respectively. Eight eyes required rebubbling due to visually significant graft detachment. While more work is needed to improve graft preparation and surgical technique, the researchers are hopeful that this method may boost endothelial graft access. “We know from previous case reports that eyes receiving only a small portion of donor tissue with healthy donor endothelial cells can clear up, demonstrating cell migration,” says Thomas John, MD, of Chicago. “If you divide one donor DM into two, you’ve already doubled your donor pool. Techniques like this may be useful, especially in parts of the world experiencing shortages of donor corneal tissue, but in the U.S., these aren’t necessarily needed at this time. Additionally, there are regulatory requirements that need to be fulfilled. Eye banks track one donor tissue to a single recipient, so segmenting grafts would require additional steps and tracking requirements. A smaller graft will also take longer to clear the cornea, since you must wait for the cells to migrate and populate the bare areas of the recipient cornea.” —CL |
Aurion Biotech, a newly launched division of CorneaGen devoted to developing Shigeru Kinoshita, MD, PhD’s cell injection therapy, announced the results of its proof-of-concept IOTA trial at the ASCRS Meeting in Las Vegas in July. Cells from two donors were used to treat 50 patients with corneal endothelial disease in El Salvador. Ed Holland, MD, the company’s chief medical advisor, reported that the minimally-invasive procedure yielded improved visual acuity and central corneal thickness.13 The company says this therapy has the potential to treat up to 100 eyes with endothelial cells from a single donor and involves a recovery period of only a few hours.
One major benefit of cell injection therapy compared to transplant procedures is that it won’t require donor-recipient matching. Human CECs are considered universal donors because the cornea is avascular; without blood present, there’s no need for a specific donor blood type, which will expand the pool of potential recipients. In the Aurion procedure, the patient’s diseased epithelium is removed by polishing; then cultured hCECs are injected into the anterior chamber and the patient lies face-down for three hours to encourage cellular adhesion to the stroma (Figure 5).
This may not be as simple as it sounds, however. “A challenge of cell injection will be localization to the inner corneal surface,” says Dr. John. “Cells may disperse into the AC angle, the TM or even the posterior segment.”
While Dr. Kinoshita’s group uses face-down positioning to encourage endothelial cell migration, others developing cell injection therapies are using alternate techniques. One potential approach to cell localization is a form of magnetic cell delivery, proposed by Jeffrey L. Goldberg, MD, PhD, a professor and chair of ophthalmology at Stanford University. He’s developed a novel method of cell delivery that involves cultured hCECs labeled with magnetic nanoparticles. These cells are injected into the anterior chamber, and the patient wears a magnetic eye patch to attract the cells toward the central cornea.14 Dr. Goldberg’s company, Emmetrope Ophthalmics, is developing the therapy. Its IND application for EO2002 was accepted by the FDA in September 2020.
Dr. Farid is a consultant for Allergan, Bausch + Lomb, BioTissue, CorneaGen, Dompe, Tarsus, Orasis, Johnson & Johnson Vision, KALA, Novartis, Sun Pharmaceutical and Zeiss. Dr. Okumura is the co-founder and a board member of ActualEyes and has received honoraria for lectures and consulting from Kowa, Astellas Pharma and Santen. He holds several pending patents related to cultured corneal endothelial cells. Drs. Colby, Busin, Yu, John, Narang, Oganesyan, Cennamo and Mencucci have no relevant financial disclosures.
1. Sarnicola C, Farooq AV, Colby K. Fuchs endothelial corneal dystrophy: Update on pathogenesis and future directions. Eye & Contact Lens 2019;45:1-10.
2. Busin M, Leon P, Scorcia V, Ponzin D. Contact lens-assisted pull-through technique for delivery of tri-folded (endothelium-in) DMEK grafts minimizes surgical time and cell loss. Ophthalmology 2016;123:476-83.
3. John T, Patel A, Vasavada A, et al. Effect of trypan blue on Descemet membrane elasticity. Cornea 2016;35:11:1401-3.
4. Majmudar PA, Johnson L. Enhancing DMEK success by identifying optimal levels of trypan blue dye application to donor corneal tissue. Cornea 2017;36:2:217-221.
5. Oganesyan O, Makarov P, Grdikanyan A, et al. Three-quarter DMEK in eyes with glaucoma draining devices to avoid secondary graft failure. Acta Ophthalmol 2020. [Epub ahead of print].
6. Woo JH, Htoon HM, Tan D. Hybrid descemet membrane endothelial keratoplasty (H-DMEK): Results of a donor insertion pull-through technique using donor stroma as carrier. Br J 7. Ophthalmol 2020;104:10:1358-62.
7. Mencucci, et al. Ultrathin Descemet stripping automated endothelial keratoplasty versus Descemet membrane endothelial keratoplasty: A fellow-eye comparison. Eye and Vis 2020;7:25. [Epub May 6, 2020].
8. Agarwal A, Dua HS, Narang P, et al. Pre-Descemet’s endothelial keratoplasty (PDEK). Br J Ophthalmol 2014;98:1181-85.
9. Dirisamer M, Yeh R, Melles G, et al. Recipient endothelium may relate to corneal clearance in descemet membrane endothelial transfer. Am J Ophthalmol 2012;154:2:290-6.
10. Koenig SB. Long-term corneal clarity after spontaneous repair of iatrogenic descemetorhexis in a patient with Fuchs dystrophy. Cornea 2013;32:6:886-8.
11. Shah RD, Randleman JB, Grossniklaus HE. Spontaneous corneal clearing after Descemet’s stripping without endothelial replacement. Ophthalmology 2012;119:2:256-60.
12. Kinoshita S, Koizumi N, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med 2018;378:995-1003.
13. Holland E. FAQ Corneal Endothelial Cell Therapy. Presented at ASCRS in Las Vegas. July 20, 2021.
14. Moysidis SN, Alvarez-Delfin K, Peschansky VJ, et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomedicine 2015;11:3:499-509.
15. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016;143:2:167-73.
16. Birbal RS, Ni Dhubhghaill S, Baydoun L, et al. Quarter-Descemet membrane endothelial keratoplasty: One- to two-year clinical outcomes. Cornea 2020;39:3:277-82.