The U.S. Food and Drug Administration approved Bausch & Lomb's single-indication orphan drug Retisert (fluocinolone acetonide intravitreal implant), 0.59 mg, for the treatment of chronic non-infectious uveitis.
B&L's Retisert received FDA Fast Track status, for priority review, and Orphan Drug designation for this indication. The company expects a mid-year launch of the product.
The patented drug-delivery microtechnology in Retisert consists of a tiny drug reservoir designed to deliver sustained levels of the anti-inflammatory corticosteroid, fluocinolone acetonide, for approximately two-and-a-half years (30 months) directly to the back of the eye.
FDA approval of the single-indication orphan drug was based on 34-week results from two three-year randomized, double-masked, multicenter clinical studies demonstrating that in eyes with Retisert there was:
• a statistically significant decrease in the recurrence of uveitis from approximately 40 to 54 percent for the 34-week period pre-implantation to approximately 7 to 14 percent for the 34-week period post-implantation;
• a statistically significant decrease in the use of adjunctive therapy including systemic corticosteroid and/or immunosuppressive therapy from approximately 47 to 63 percent at the time of implantation to approximately 5 to 10 percent at 34 weeks post-implantation, and for patients needing periocular corticosteroid injections from approximately 50 to 65 percent for the 34- week period pre-implantation to approximately 3 to 6 percent for the 34- week period post-implantation;
• statistically significant improvement of three or more lines of visual acuity in approximately 19 to 21 percent of study eyes at 34 weeks post-implantation.
The most common adverse events include cataract progression, increased intraocular pressure, and procedural complications and eye pain. For full prescribing information, visit bausch.com.
Two-year results of a randomized, double-masked, multicenter controlled clinical trial of Retisert were scheduled to be presented at the annual meeting of the Association for Research in Vision and Ophthalmology in Ft. Lauderdale, Fla.
The FDA also approved the ReZoom multifocal refractive IOL for cataract patients from Advanced Medical Optics Inc.
|
|
| AMO"s ReZoom Multifocal IOL |
Also approved was Alcon's AcrySof ReSTOR IOL for cataract patients with and without presbyopia. This lens uses an apodized diffractive technology to give patients a full range of near, intermediate and distance vision. The clinical studies supporting the approval showed that 80 percent of patients who received the AcrySof ReSTOR lens did not use glasses for any activities after cataract surgery.
The clinical results also showed that 84 percent of patients who received the AcrySof ReSTOR lens in both eyes achieved distance visual acuity of 20/25 or better and near visual acuity of 20/32 or better without correction by contacts or glasses while only 23 percent of the conventional or monofocal control group achieved this level. Near visual acuity of 20/32, or J2, means patients can read the very small stock quotes in the newspaper.
"Approval of the AcrySof ReSTOR lens is a significant event for Alcon that validates the extensive development work we have done to make it the best lens possible for all of a patient's vision needs. We expect AcrySof ReSTOR to be a contributor to our growth in the coming years," said Cary Rayment, president and CEO of Alcon.
New ISTA NSAID Approved
The agency also approved ISTA Pharmaceuticals' Xibrom (bromfenac ophthalmic solution) 0.09% for the treatment of ocular inflammation following cataract surgery. ISTA expects to launch Xibrom, a topical, twice-daily, non-steroidal anti-inflammatory solution, during the second quarter of 2005, after securing commercial quantities of the product from its manufacturer and completing the further expansion of its sales force. Eric Donnenfeld, MD, an investigator in the Xibrom Phase III clinical trials, commented, "Xibrom is the first twice-daily ophthalmic NSAID to be approved in the United States. All other ophthalmic NSAIDS are dosed four times daily. Xibrom represents an advance for ophthalmic care because of the improved patient compliance and its early onset of action, and I am pleased that patients will have this new treatment option." Vicente Anido Jr., PhD, president and CEO of ISTA stated, "We are excited to receive FDA approval of our third commercial product and anticipate launching Xibrom during the second quarter of 2005."
Senju Pharmaceuticals Co. has marketed Xibrom in Japan since 2000. ISTA acquired U.S. marketing rights in May 2002. In two Phase-III studies involving 527 patients, a statistically significant proportion of patients treated with Xibrom achieved treatment success, defined as the complete absence of ocular inflammation, compared to those patients who received placebo. This effect was evident in the Xibrom group as early as day three following initiation of treatment. ISTA filed its NDA for Xibrom with the FDA in May 2004.
Moria's Epi-K System Approved
The FDA ALSO approved Moria's Epi-K device for use with the new Epi-LASIK refractive procedure.
The Epi-K system uses a metal epithelial separator encased in a disposable plastic head. The company says this design helps ensure that the blade doesn't make contact with other metal surfaces, accidentally become sharp, and then cut stroma instead of just separating epithelium from stroma. The system also uses an applanation plate to keep the separator from cutting down into the stroma. Not cutting into stroma is key to avoiding potential scarring and irregular outcomes in the epi-LASIK procedure, in which the epithelium is separated from stroma using a separator, and a hinge is created. The epithelial flap is folded to the side; the surgeon then performs the ablation, and puts the epithelial sheet back in place.
The design of the Epi-K is similar to the company's One-use Plus and M2 LASIK keratomes. The main differences are in the handpiece motor and the blade. The motor moves the head more slowly than in LASIK, with a corneal pass lasting 14 seconds compared to just two or three as in LASIK.
Mark Swanson, MD, who practices in Agua Prieta, Sonora, Mexico, took part in a study of the Epi-K device. In the study of 40 cases, the patients' average preop myopia was -4.5 D, and they were otherwise operable with either LASIK or PRK. The average preoperative horizontal K-reading was 43.74 D, with an average vertical K-reading of 44.61 D.
Postop, the average size of an attempted 9-mm flap was 9.2 mm, with an average thickness of 52.3 µm (SD=9.3 µm). The average hinge size was 4.7 mm (SD=1.3 mm). Also, 97.5 percent of flap edges were graded as excellent (no edge imperfections), says Dr. Swanson, and 97.5 percent of the eyes had excellent stromal beds. One eye had partial thickness retained epithelium. However, there were no cases of stromal cuts or reverse buttonholes in the study, and Dr. Swanson says that now, after having done around 300 cases with the Epi-K, he has yet to cut into stroma with the device.
"The device has helped us treat patients in whom there would be a little risk by doing LASIK based on their topography or pachymetry readings," says Dr. Swanson. "Their corneas may be thin, or thinner than normal, and we don't want to risk ectasia developing. With epi-LASIK, we don't have to cut into stroma. We just cut the epithelium and do the surface ablation … the flap it makes looks like a LASIK flap. The flap's borders are well-defined. It's an impeccable flap."
The Epi-K system costs $67,000, and comes with the Evolution 3E control unit, which has a multi-speed foot pedal specifically used with the Epi-K, two Epi-K handpieces, and four reusable metal rings. The disposable heads cost $85 each.
Moria is currently filling orders for surgeons who pre-ordered the Epi-K, and plans to finish fulfilling these requests by the end of May. It then plans to start filling new orders for the device. According to Moria, owners of previous versions of the Evolution control unit who wish to use the Epi-K can trade in their old units for a "generous" trade-in allowance.
A study by a group at Washington University School of Medicine in St. Louis is shedding new light on the role oxygen plays in cataract development.
As reported in the American Journal of Ophthalmology, they measured oxygen concentrations in the eyes of patients undergoing retinal surgery. "It's fairly well-accepted in the field that anyone over the age of 50 who has vitrectomy surgery will develop a cataract within two years," says Nancy M. Holekamp, MD, of the Barnes Retina Institute, and the study's lead author. Just before surgery, Dr. Holekamp measured oxygen levels adjacent to the lens and near the center of the eye in the vitreous gel of 69 eyes. Before retinal surgery, oxygen concentrations were very low in both places. After surgery, oxygen levels in both locations were about eight times higher than normal.
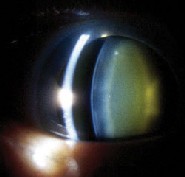 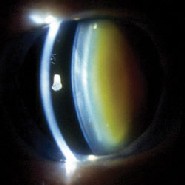 |
| This eye of a 72-year-old woman shows normal yellowing of the lens (left) due to age-related nuclear sclerotic cataract. The same woman's other eye shows opacification due to more severe nuclear sclerotic cataract two years post-vitrectomy. |
Even after vitrectomy, Dr. Holekamp says, it may be possible to lower the amount of oxygen near the lens by lowering the oxygen level in the fluid that is pumped into the eye. "We're proposing that we deoxygenate the fluid used to replace the vitreous gel," she says. "There's no good reason to infuse such a highly oxygenated fluid into the eye. In fact, if we want to perform surgery under more natural conditions, we should remove the oxygen from that fluid."
A co-investigator, David C. Beebe, PhD, believes the same kind of mechanism may contribute to cataracts that form as people age. The difference is that in age-related cataracts, the gel breaks down over several years. In vitrectomy patients, the gel disappears all at once.
Dr. Beebe and colleague Ying-Bo Shui, MD, PhD, have demonstrated a statistical relationship between breakdown of the vitreous gel and the risk for cataracts. They believe that when the gel separates from the retina or begins to break down and liquefy, it allows fluid to flow over the surface of the oxygen-rich retina and carry that oxygen to the lens. That's true whether the cause is vitreous liquefaction or the vitreous gel has been removed.
In past research, Dr. Beebe and his colleagues examined eye-bank eyes and found that the vitreous gel was almost completely intact in some eyes. The incidence of nuclear cataracts was very low in those eyes. But in eyes in which the vitreous gel had broken down, there was a much higher incidence of cataracts.
The work suggested that some retinal diseases may protect patients from cataracts. For example, patients who had retinal surgery for complications of diabetes had significantly lower levels of oxygen near the lens. They plan a follow-up study of whether diabetic patients are somehow protected from cataracts.
They also note that researchers in Japan have performed retinal surgery without removing the vitreous gel. The surgery is more difficult to do, but the Japanese team found that when the vitreous gel remains intact, retinal surgery patients don't develop nuclear cataracts to the same extent.
Dr. Holekamp isn't proposing that retinal surgeons stop doing vitrectomies, but she says lowering oxygen concentrations in the fluid that replaces the vitreous gel might help protect the lens. She and her colleagues plan to investigate methods to do so. Dr. Beebe, too, is planning to conduct animal studies to see if he can prevent oxygen from reaching the lens.
"If we can remove or reduce the amount of molecular oxygen that reaches the lens, and that turns out to protect against nuclear cataracts in either human patients or in animals, I think it will make a very strong case for oxygen being the culprit," he says.
"Perhaps we could replace the vitreous gel with a gel polymer that would keep oxygen away from the lens," he says. "Now that we're beginning to get an idea of how the vitreous gel works, it may be possible to design interventions to protect the lens both in people who have had a vitrectomy and in those whose vitreous is degenerating as a part of normal aging."
Am J of Ophthalmol 2005;139:302-310.
|
Correction |
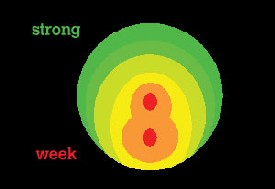
| In the March 2005 article, "Refractive Surgery: Insights on Ectasia," the labels on the image on p. 34 showing the "Strong" and "Weak" aspects were reversed. The correctly labeled figure and caption appear here. Review of Ophthalmology regrets the error.
|