Spectralis HRA+OCT is a spectral domain system, sometimes called fourier domain, which scans the retina at 40,000 scans per second, creating highly detailed images of the structure of the retina. Because the OCT and HRA images are captured simultaneously, the clinician can be assured of the exact location of the area of interest and can correlate the outer visible retina structure with the internal structure. The new product is built on the company's successful Heidelberg Retina Angiograph (HRA) platform, the first commercial angiography system to use lasers in combination with marker dyes such as sodium fluorescein and indocyanine green (ICG). Using the HRA instead of white light photography has allowed clinicians to capture detailed images of the blood vessel structure within the retina, a key diagnostic indicator for such common eye disease as age-related macular degeneration and diabetic maculopathy. Another advantage of using lasers is the fast frame rate which enables movies of the blood flow, adding a new diagnostic dimension over traditional photography. Of recent interest is the HRA's capability to cause certain retinal components to fluoresce in a process known as autofluorescence. Geographic atrophy of age-related macular degeneration is being followed using autofluorescence as a potential early indicator of disease progression in the recently announced AREDS 2 clinical trial. The company expects to begin shipping the product in mid-2007.
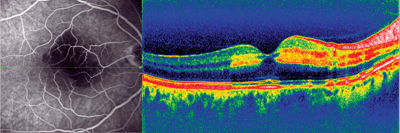
Arterial occlusion and embolus captured on the new Spectralis HRA+OCT.
ISTA Licenses Senju's Allergy Treatment
ISTA Pharmaceuticals has licensed the exclusive North American rights to an eye-drop formulation of bepotastine from Senju Pharmaceuticals Ltd. The eye drop is an investigational ophthalmic treatment for the treatment of allergic conjunctivitis, ISTA says.
Bepotastine has been approved to treat allergic rhinitis and uriticria/puritus for patients in Japan for several years. It is a non-sedating, selective antagonist of the histamine 1 (H1) receptor and has a stabilizing effect on mast cells, ISTA says; it suppresses the migration of eosinophils into inflamed tissues. ISTA anticipates moving bepotastine into Phase III clinical studies in the U.S. in early 2007. For information visit istavision.com.
New from FCI Ophthalmics
FCI Ophthalmics has a pair of new introductions. The Mono-Crawford combines the Monoka and the Crawford lacrimal intubation systems. One end of the stent features a silicone plug that anchors the system securely in place at the punctum. The other end is an olive-tipped probe that facilitates retrieval from the nose using the Crawford hook (also available from FCI).
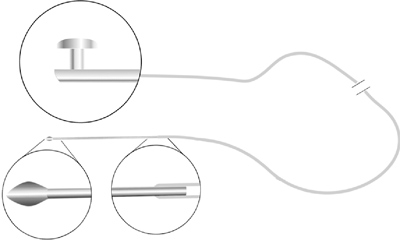
The Mono-Crawford is appropriate for congenital nasolacrimal duct obstruction, laceration, or in cases in which only one punctum is functional. The stents come with either medium (3 mm) or wide (4 mm) collarettes and are available with a PVP coating to enhance tear drainage.
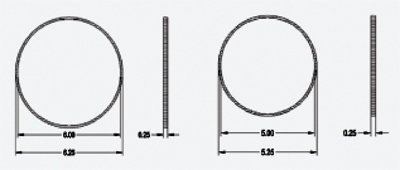
The Morcher Ring Caliper is a sterile, temporary surgical device that enables the surgeon to create a more precise anterior capsulorhexis in primary or secondary IOL exchange procedures and is recommended for any surgical procedure that requires a calibrated anterior capsulorhexis of a precise diameter. It's available in Type 5 (inner diameter 5.0 mm) or Type 6 (6.0 mm).
Because the Ring Caliper is placed directly on top of the anterior capsule, the patient's eye movement does not interfere with the capsulorhexis. The Ring Caliper is easily inserted through the incision site and can be positioned with an Osher Manipulator or Kuglen Hook. Call 1 (800) 932-4202 or visit fci-ophthalmics.com.
Second Generation PHP
The Foresee PHP, the second generation Preferential Hyperacuity Perimeter is now available for improved detection and early diagnosis of age-related macular degeneration. The Foresee PHP is the follow-up to the Preview PHP, the only ophthalmic device to receive FDA clearance for monitoring the progression of AMD. The system allows eye care professionals to track any changes to a patient's visual field resulting from the advancement of the disease. The updated device is completely automated, allowing for patient self-operation, while still exhibiting high sensitivity and specificity, according MSS Ophthalmic Services and Equipment, a subsidiary of TLCVision Corp. The Foresee PHP is a non-invasive, easy-to-perform eye exam that has proven highly effective in identifying elevations in the retinal pigment epithelium that are consistent with conversion from intermediate to wet AMD. Its diagnostic capability is based on hyperacuity, the inherent human ability to visually detect the misalignment of objects relative to other objects in space. Hyperacuity is 10 times more sensitive than standard visual acuity and is characteristically stable regardless of a patient's age or physical condition.
For information, e-mail info@ms-services.com or call MSS at 1-800-728-9615 .
IOL Education Software
Ocutouch 360 All-Office Education Software, from Trevi Technology, features seven patient education programs for use throughout the ophthalmic practice including the waiting room, exam and consult areas, and the dispensary.
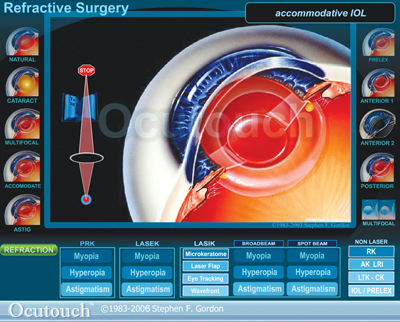
Version 3.0 includes a new interactive IOL Comparison and Demo Program that explains and demonstrates the different sizes and shapes of various IOLs and how they work to correct vision and astigmatism. Animated sequences depict accommodative IOLs, phakic posterior and anterior designs, and multifocal and prelex lenses. Visit trevitechno logy.com or call 1 (888) 668-7384.



