The Dutch Ophthalmic Research Center received FDA approval of its New Drug Application for TissueBlue (Brilliant Blue G Ophthalmic Solution) 0.025%. TissueBlue is the first FDA-approved dye for use as an aid in ophthalmic surgery by selectively staining the internal limiting membrane. The stain is injected onto the inner retinal surface for clear staining of the ILM as a way to distinguish it from unstained retina during its removal. Additional highlights include:
• its use with more than 350,000 surgical procedures since launch;
• a unique U.S. formulation featuring pharmaceutical-grade dye material to ensure a higher level of purity than lower-grade (compounded) dyes;
• the inclusion of polyethylene glycol to provide the density required by surgeons to ensure targeted application to the retina; and
• availability in a terminally sterilized, prefilled syringe.
DORC says it expects to start shipping TissueBlue to customers in early April. Visit www.dorc.eu/products for more information.
B+L Expands Parameters for Biotrue OneDay for Astigmatism
Bausch + Lomb recently announced the U.S. launch of expanded parameters for Biotrue OneDay for Astigmatism daily disposable contact lenses. The expansion will increase the toric parameter range by more than 60 percent, the company says. Similar to the other products in the Biotrue OneDay brand family, Biotrue OneDay for Astigmatism lenses are formulated with a patented dehydration barrier, which B+ L says helps the lens maintain nearly 100 percent of its moisture for a full 16 hours. An evolved peri-ballast design features a tapered edge to limit lid interaction, and spherical aberration control to help reduce halos and glare, particularly in low-light conditions. Visit www.bausch.com.
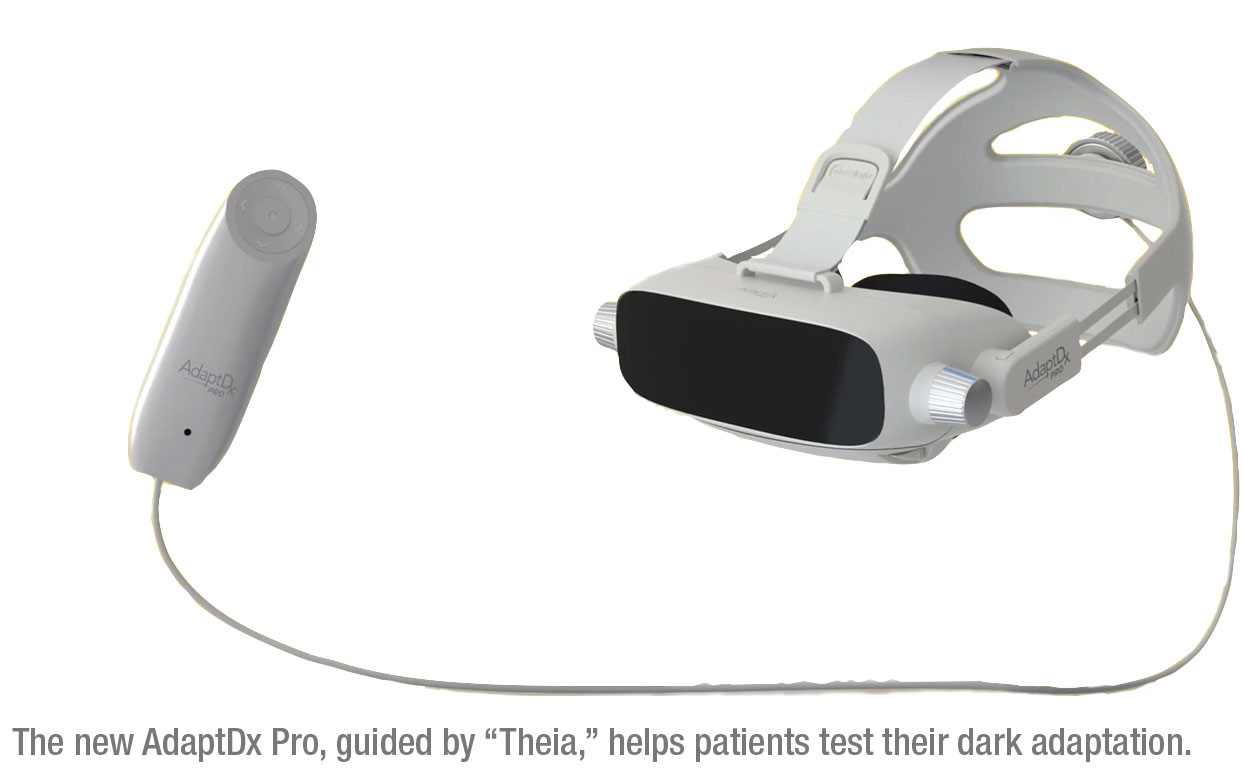
Meet Theia, An Alexa for Your Eyes
MacuLogix announced its next generation of dark adaptation functional testing with the introduction of AdaptDx Pro, guided by “Theia.” A new headset, custom-designed and tested for patient comfort, includes all of the functionality and accuracy of the company’s table-top dark adaptometer, the company says. As a self-contained wearable headset, the AdaptDx Pro requires no darkroom or external computer, and features an artificial intelligence-driven onboard technician named Theia. After the in-office technician selects the testing protocol and places the device on the patient’s head, Theia takes over to facilitate the testing experience by using automated instructions and adaptive feedback spoken directly to the patient. Theia’s A.I. helps ensure consistent, reliable testing results, and frees up the technician to focus on other tasks, MacuLogix says. Visit www.maculogix.com/adaptdx/. REVIEW
 |