|
The U.S. Food and Drug Administration recently approved faricimab, now known as Vabysmo (faricimab-svoa, Genentech/Roche), for the treatment of nAMD and DME. The Phase III nAMD trials (TENAYA and LUCERNE, n=1,329) and DME trials (YOSEMITE and RHINE, n=1,891) comparing intravitreal faricimab 6 mg to aflibercept 2 mg, all met their primary endpoints. “This drug works as well and is as safe for the two most common disease states for which we administer anti-VEGF therapy,” says Wills Eye Hospital’s Carl Regillo, MD, FACS, a clinical investigator for the Phase III trials for both indications and consultant for Genentech. “Its extended durability means fewer total treatments will be needed to control disease in a comparable way.”
Faricimab is a bispecific antibody that simultaneously binds both angiopoietin2 and VEGF-A. By targeting two pathologic growth factors, the drug may provide a better or more durable effect, explains Dr. Regillo.
“There’s a lot of pre-clinical evidence to suggest that Ang2 plays a pathologic role in disease states of the retina—both in wet AMD and DME,” he says. “In disease states, Ang2 is upregulated and appears to work in conjunction with the upregulation of VEGF-A in promoting vascular instability with leakage and new blood vessel formation—the pathologic signs we see clinically in these conditions. Blocking both factors has a potential advantage over blocking only VEGF-A.”
For both nAMD and DME, patients receive four loading doses, followed by maintenance dosing every two, three or four months for nAMD and every one to four months for DME, depending on anatomical and visual outcomes. The drug was also approved for a second DME treatment regimen consisting of six monthly loading does and maintenance treatment every two months. Monthly treatments for nAMD and DME are approved, if needed, but the studies didn’t find additional efficacy at this dosing frequency.
In both the Phase II and Phase III trials, faricimab was well-tolerated and demonstrated safety comparable to ranibizumab and aflibercept. “For both nAMD and DME, faricimab dosed in a less-frequent regimen (q8, q12 or up to q16 weeks) during the maintenance phase produced noninferior vision gains compared to aflibercept dosed on-label,” Dr. Regillo says.
Additionally, he says faricimab demonstrated better drying than aflibercept in DME. “That’s particularly relevant in DME because studies indicate there’s some correlation between better drying and better visual outcomes for some patients,” he notes. “Aflibercept enjoys the reputation of being a bit more effective visually with better drying in DME, so the faricimab outcomes exceeded our expectations. Similarly, when faricimab was compared to ranibizumab in the Phase II trial, it also showed trends indicating better drying and durability.”
Dr. Regillo says the rates of intraocular inflammation in all four Phase III trials were consistent and about the same as those among aflibercept-treated patients. “Faricimab rates were numerically slightly higher in both programs, but these rates were still low, in the 1- to 2-percent range. This is similar to what we’ve seen in clinical trials with anti-VEGF biologics over the years. We didn’t see any concerning adverse events of special interest such as retinal vasculitis or inflammation-related vascular occlusion.
“For a drug to perform as well both in efficacy and safety, and to last longer, helps to decrease the treatment burden, which is a major unmet need,” Dr. Regillo continues. “Our anti-VEGF biologics that we’ve been using for years work really well, but they must be administered frequently to get optimal long-term vision outcomes for both nAMD and DME. It’s really hard to do that in practice. Real-world studies show that we’re not able to sustain the vision gains we get upfront after the initial loading phase very well, because we simply can’t provide the number of treatments per year that we need to in order to keep these conditions under optimal control. It’s a burden on patients, who must make time to come to the office for their injections. We hope that in the long-run, more durability will translate into better maintenance of vision gains, and therefore better long-term vision outcomes.
“It’s a big year to have this drug approved simultaneously for both nAMD and DME,” Dr. Regillo says. “We welcome the increased durability. I think we’re all expecting it to be utilized quite a bit over time.”
 |
|
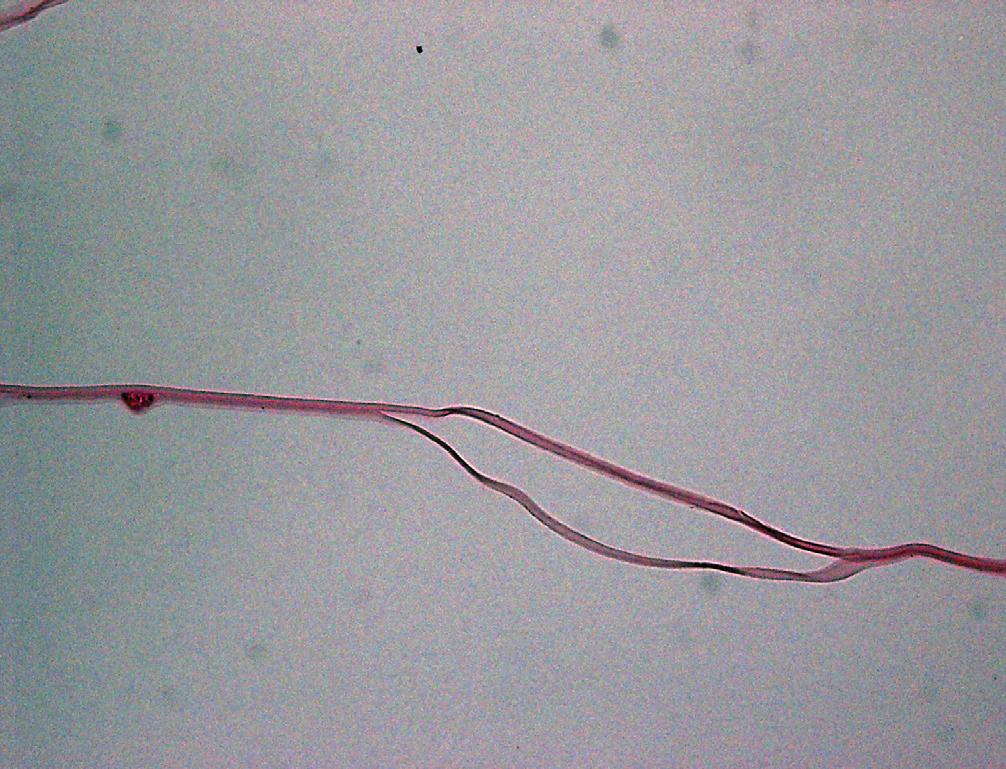
Light photomicrograph of a histopathological section cut from a capsular bag from a suspected dead bag case, showing capsular splitting/delamination. Image courtesy of Liliana Werner, MD, PhD, John A. Moran Eye Center, University of Utah. |
What’s Behind “Dead Bag” Syndrome?
In a case-series study, researchers recently set out to more fully describe the recently recognized phenomenon known as “dead bag” syndrome, in which the capsular bag appears to be clear for years following cataract surgery, but then becomes diaphanous, floppy and unable to support the intraocular lens that was implanted.
In the study of 10 cases that the surgeons suspected to represent a dead bag syndrome, eight IOLs and seven capsular bags were removed because of subluxation or dislocation. The researchers fixed the seven bags available for analysis in formalin and submitted them to histopathological examination (hematoxylin-eosin and Masson trichrome stains). They also examined the explanted IOLs in five cases.
Histopathologically, the seven capsular bags showed capsular thinning and/or splitting. Lens epithelial cells were completely absent on two specimens, whereas the other five specimens had rare LECs on the inner surfaces of their capsules. Explanted IOLs were three-piece silicone IOLs or single-piece hydrophobic acrylic IOLs. One IOL optic showed a small amount of granular pigment deposition, but the optics of the other four IOLs were unremarkable, the researchers say.
The authors say that, in this syndrome, there seems to be an absence of secondary proliferation of LECs and fibrotic changes. The capsule shows some signs of degradation, such as thinning and/or splitting. Weakness of zonular attachments seems to be an associated finding, with subsequent in-the-bag IOL dislocation. “Although the dead bag syndrome specimens reported in this study share characteristics with both true exfoliation and pseudoexfoliation, there are specific differences that show dead bag syndrome to be a distinct entity,” the researchers say. They add that further studies will be required to pin down the etiology of the condition.
1. Culp C, Qu P, Jones J, et al. Clinical and histopathological findings in the dead bag syndrome. J Cataract Refract Surg 2022;48:2:177-184.
Industry NewsThea Acquires Select Akorn Products Théa SAS, based in Clermont-Ferrand, France, entered into an agreement to purchase seven branded ophthalmic products from Akorn. Once the transaction with Akorn is completed, its portfolio will comprise brands including Zioptan, AcellFx, Cosopt, Cosopt PF and Azasite. Opthea Announces OPT-302 Data in PCV Opthea announced that findings on OPT-302 were presented at the annual Angiogenesis, Exudation, and Degeneration annual meeting, held virtually. OPT-302 combination therapy had a safety profile consistent with standard of care anti-VEGF-A monotherapy, and demonstrated greater improvements in best-corrected visual acuity and less retinal fluid compared to ranibizumab monotherapy. EyePoint Announces Updated Interim Data from Phase I DAVIO EyePoint Pharmaceuticals announced updated interim data from the DAVIO Phase I clinical trial of EYP-1901, a bioerodible sustained-delivery intravitreal anti-vascular endothelial growth factor treatment targeting wet age-related macular degeneration, at the Angiogenesis, Exudation, and Degeneration annual meeting. The interim eight-month follow-up data presented from the Phase 1 DAVIO clinical trial continue to show no reports of ocular or drug-related systemic serious adverse events, the company says. Eyenovia Receives FDA Guidance on MydCombi NDA Resubmission Eyenovia announced that the company completed a Type A meeting with the FDA related to the refiling of the NDA for MydCombi, the company’s combination of tropicamide and phenylephrine for in-office pupil dilation. In 2021, Eyenovia received a complete response letter from the FDA stating that MydCombi had been reclassified as a drug-device combination product. The FDA requested the company conduct additional device testing related to the Optejet dispenser, but no additional clinical studies of MydCombi were requested. |
Surgeons Shaking Off COVID-19 Rust
During the pandemic, elective cataract surgery has been significantly curtailed. In an effort to investigate whether a consequent reduction of surgical skill led to higher operative complication rates, researchers recently found that following nine months of curtailed elective cataract surgery, posterior capsular rupture rates were increased across all surgeon grades, with similar risk percentage scores.1 Cystoid macular edema rates were also increased, unrelated to the proportion of diabetics or increased PCR rates.
The researchers note that, after six months, de-skilling, particularly of fine-motor skills, is rapid and followed by a slower skill degradation with time, with the rates of deskilling varying between individuals, due to mitigating factors such as stress, anxiety and lost confidence.
This single-center study evaluated consecutive patients undergoing cataract surgery during three periods:
P1: prior to the pandemic (February 1, 2019 to January 13, 2020)
P2: after the first lockdown (June 3, 2020, to January 11, 2021
P3: during/after the second lockdown (January 25, 2021, to July 30, 2021)
A total of 2,276 operations occurred during P1, 999 during P2 and 846 during P3. During P1, the PCR rate was 1.7 percent, similar to P2 (1.3 percent) but lower than P3 (3.6 percent). There was no difference in PCR risk percentage score between routine and PCR cases during P1 (1.9 percent vs 2.0 percent), P2 (2.0 percent vs 2.2 percent) or P3 (1.9 percent vs. 2.7 percent).
During P2 and P3, there was a higher rate of CME compared with P1 (4.9 percent and 6.9 percent, respectively, vs. 1.9 percent), with no difference in proportion of diabetics or cases with CME in combination with PCR. There was no difference in surgeon grade experiencing PCR.
“With the knowledge that extensive breaks from surgery lead to skill fade, it would seem very sensible that ophthalmology attempts to investigate factors that may optimize returns after surgical breaks,” the study authors concluded in their paper. “Clearly, there is a need—to improve patient safety—for more support for surgeons of all grades when they return to surgery after an extended hiatus, with the development of robust guidelines, including, perhaps, mandatory time spent on surgical simulators.”
1. Theodoraki K, Naderi K, Lam CFJ, et al. Impact of cessation of regular cataract surgery during the COVID pandemic on the rates of posterior capsular rupture and post-operative cystoid macular oedema. Eye (Lond). February 3, 2022. [Epub ahead of print].