Scientists at the
In a recent study, two researchers from the
Because each woman's disease was in a very early stage, the researchers could evaluate how accurately the instrument would detect vision loss as compared to several standard tests used to evaluate vision: visual field, visual acuity, and pupillary light response. In each case the imaging instrument provided results that were equal to and often superior to the standard tests.
The study grew out of Dr. Petty and Dr. Elner's observation that metabolic stress at the onset of disease causes certain proteins to become fluorescent. To measure the intensity of this flavoprotein autofluorescence, they designed a unique imaging system equipped with state-of-the art cameras, filters, and electronic switching, together with customized imaging software and a computer interface.
Dr. Petty, a biophysicist and expert in imaging, says "Autofluorescence occurs when retinal cells begin to die, often the first event in diseases like glaucoma and diabetic retinopathy. Cell death can be observed microscopically, but not as yet through any current imaging methods. We believe this study is a big step forward toward creating a diagnostic tool that can characterize disease long before symptoms or visible signs appear."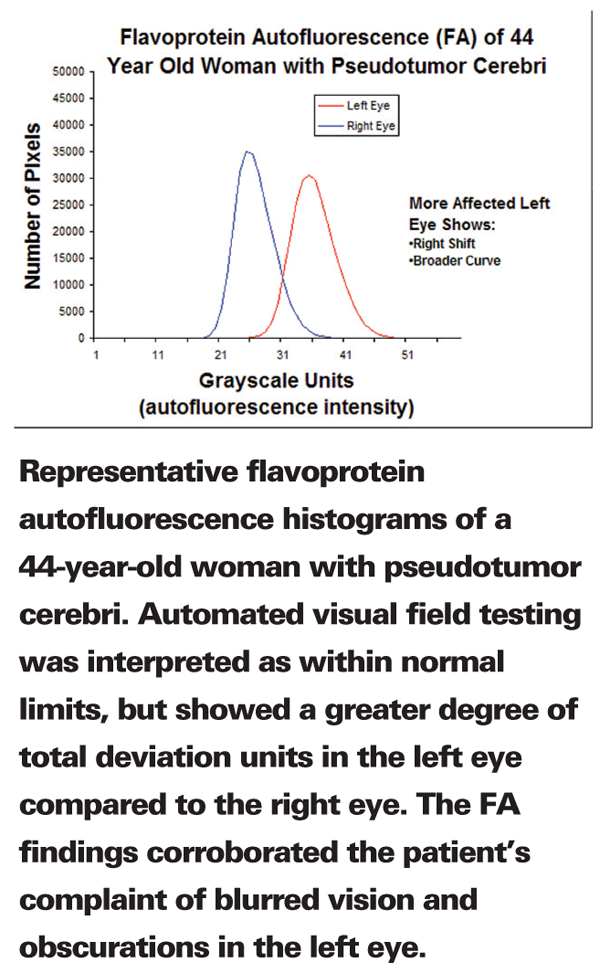
The women in the study were newly diagnosed with PTC and had not yet received treatment. According to standard tests they had good visual acuity, and their visual field tests indicated only subtle abnormalities or none at all. The researchers measured FA values for the six women and an age-matched control group. All of the patients with PTC had higher FA values in the eye that was more severely affected. In fact, FA values averaged 60 percent greater in the more-affected eye of these women. By contrast, the control group had no significant difference in FA values between their healthy eyes.
The researchers also found that FA data more accurately described the different degree of disease in each eye for a given patient, as compared to the standard vision tests.
Dr. Elner, an ophthalmologist and a pathologist, says "Early treatment for eye disease is so important, and this study suggests that FA activity is a very good indicator of eye disease. Cardiologists have long used blood pressure testing to head off heart disease. We believe that FA testing will likewise be a helpful diagnostic tool for eye doctors looking to prevent blindness."
Drs. Elner and Petty have patented the device, and they are investigating its use as a screening device in other major eye diseases as well as in diabetes.
Role Identified for Glaucoma Gene and Signaling Pathway
A team of researchers from Alcon Research, the
Their study, which revealed that over-expression of the gene, sFRP1, elevates pressure in the eye, could help improve glaucoma diagnosis and lead to the development of sight-saving treatments.
"The cause of glaucoma and the resulting elevation of intraocular pressure has been poorly understood," said Abe Clark, PhD, Alcon's vice president of discovery research and head of glaucoma research. "This new discovery may allow researchers to develop therapies to treat the underlying cause of the disease."
"Although there have been leaps and bounds in glaucoma research, we are just beginning to understand the causes of high pressure in the eye and nerve damage that leads to vision loss in glaucoma," said study team member John Fingert, MD, PhD, assistant professor of ophthalmology and visual sciences at the UI Roy J. and Lucille A. Carver College of Medicine.
Jeffrey Rubin, MD, PhD, at NCI's Center for Cancer Research, who was involved in the study, had previously discovered the sFRP1 gene. The team compared the genes that are expressed in the eyes of people with glaucoma to the genes that are expressed in people with healthy eyes. They saw that some genes, including sFRP1, are much more active, or "expressed," in cells from eyes with glaucoma. sFRP1 is part of a signaling pathway involving a series of other genes known as the WNT-signaling pathway. The team tested the effects of the gene on the pressure in both human donor eyes and mouse eyes. When the investigators delivered sFRP1 protein to the eyes, the pressure in these eyes became elevated.
The results suggest that over-expression of sFRP1 disrupts the WNT-signaling pathway and seems to cause glaucoma's hallmark high pressure in the eyes. In addition, the team found that applying a substance that normalizes the WNT-signaling pathway significantly reduced high pressure in mouse eyes; this was not tested in human donor eyes.
"We are hopeful that further study of sFRP1 and the WNT-signaling pathway will help advance our understanding of why some people get glaucoma and others do not," Dr. Fingert said. The results of the study, which was funded by Alcon Research, appeared online Feb. 14 in the Journal of Clinical Investigation.
SRT Laser Part of Stem Cell Study
Stemedica Cell Technologies Inc., which manufactures adult stem cells, has entered into an agreement with laser manufacturer Lumenis to develop a comprehensive clinical study for the treatment of macular degeneration using Stemedica's MCT (Multiple Cell Technology) adult stem cells and the Lumenis SRT Laser. The clinical study will be conducted at the Fyodorov Eye Institute in
The clinical studies will focus on combining stem cell therapy with the use of the Lumenis SRT laser to treat macular degeneration. The year-long study will begin in March 2008 and will determine the safety and efficacy of the use of Stemedica's proprietary MCT stem cell lines in combination with the Lumenis SRT laser. "We are excited about the potential of this combination therapy," said Dr. Paul Frohna, Stemedica's vice president of Regulatory & Clinical Affairs. "We believe that Stemedica's technology together with Lumenis' new laser technology holds great promise for successfully treating patients who otherwise have no treatment options."
Casey Eye Institute To Study Stem Cells For Retinal Disease
StemCells Inc. and the Oregon Health & Science University Casey Eye Institute have entered into a research collaboration under which the institute will evaluate the company's proprietary HuCNS-SC (purified human neural stem cells) product candidate as a potential treatment for retinal degeneration. Published studies conducted with rat models have shown that human neural stem cells can protect retinal function and thereby preserve vision. The research, preparatory to planned clinical trials, is expected to be finished by the end of the year.
For more information, visit stem cellsinc.com.
Symposium Seeks New Outcome Measures
The National Eye Institute and the Food and Drug Administration will sponsor a symposium in March to consider new disease-relevant outcome measures appropriate for evaluating glaucoma therapies. Currently, clinical drug trials for glaucoma therapies rely on standard perimetric criteria (i.e., a vision field test) as the primary functional outcome measure. However, new technologies that assess abnormalities and changes in the optic nerve structure and its function offer vision researchers alternative pathways to better diagnosis and treatment. The symposium will focus on new outcome measures appropriate for evaluation of glaucoma therapies, with the aim of encouraging the development of new therapies, facilitating their evaluation and, ultimately, benefiting patients. Attendees will include clinical researchers/basic scientists, clinical trial specialists, pharmaceutical company representatives, association representatives, members of legal/advocacy firms and biotech company representatives. Registrants will be able to submit questions and comments in advance through a link on their registration confirmation notices. The symposium is managed by the Association for Research in Vision and Ophthalmology.
NSAID Treatment May Reduce Number of Lucentis Injections
An abstract to be Presented at this year's ARVO meeting suggests that the use of a non-steroidal anti-inflammatory agent may improve the results of Lucentis treatment.
The study combined Xibrom (bromfenac ophthalmic solution) 0.09% therapy with Lucentis (ranibizumab injection) in patients with AMD. According to the abstract, patients who received Xibrom required 1.6 ±0.69 injections of Lucentis during the six-month study period, while patients who received only Lucentis received 4.5 ±0.41 injections. Patients with Lucentis alone received significantly more (2.83 times) injections of ranibizumab (p=0.0002) than those receiving the combination. There was a numerical trend in favor of the combination treatment group on improvement in visual acuity but this difference did not achieve statistical significance.
The study results were gathered from 60 patients receiving Lucentis therapy for wet AMD. Patients were monitored monthly using optical coherence tomography and fluorescein angiography. When leakage from vessels was detected, patients were re-injected with Lucentis. Thirty patients received Xibrom dosed twice daily in addition to the Lucentis injection, and their results were compared to 30 patients who received Lucentis only. There were no adverse events associated with the extended topical administration of bromfenac. The study was designed as a retrospective case control series.
The poster (#A531) will be presented on April 27, 2008 at 11 a.m. by Calvin A. Grant, MD. The study was supported by an unrestricted research grant from Ista. The full abstract is available at arvo.org.



