A recent study utilizing a patent-pending formulation of zinc and phytase (trademarked as Zytase), demonstrated that increasing zinc levels by oral administration for four days prior to a planned injection of botulinum toxin improved efficacy and/or duration in 41 of 44 patients tested (93 percent). Phytase was added to increase zinc absorption and significantly improved outcomes.
Oral supplementation of Zytase prior to administration of botulinum toxin effectively increased responsiveness in virtually every individual tested. Many of these patients were considered to be poorly responsive to botulinum toxins such that control of their blepharospasm symptoms had been historically difficult to achieve. The results showed that Zytase supplementation resulted in a remarkably effective treatment of blepharospasm using the same botulinum toxin source and dosage that was previously less effective in the same patient in the absence of supplementation.
Among other things, the results suggest that lowered doses of botulinum toxin could be administered with reasonable responsiveness in some patients if Zytase is supplemented prior to treatment. Intake of zinc/phytase supplementation prior to administration provided increased responsiveness which can be normalized across the patient population and one important variable in individual responsiveness can be eliminated.
Finally, by supplementing with Zytase prior to botulinum toxin injections, results for blepharospasm patients were enhanced while prolonging the duration of treatments.
Charles Soparkar, MD, PhD, and the research team will present the effect of dietary zinc supplementation on botulinum toxin treatments at the American Society of Ophthalmic Plastic & Reconstructive Surgery 41st Annual Fall Scientific Symposium on October 14 in
Zytase has been licensed to Ocusoft. To eliminate the potential for patient abuse and resulting zinc toxicity, Zytase will be marketed as a prescription when commercially available in September 2010. Zytase will be packaged in a blister pack of ten capsules ensuring both its Rx designation and the quantity of capsules so that physicians will remain in control at all times. For information, visit ocusoft.com.
B+L Releases Single-Use Peeling Forceps
Bausch + Lomb Storz Ophthalmic Instruments has released a new, single-use, asymmetric peeling forceps for vitreoretinal surgery. The forceps are available in 23- and 25-ga. options for facilities seeking the safety, consistency and convenience a single-use instrument offers.
The peeling forceps feature an asymmetric jaw design for excellent visualization of retinal membranes with a sleek, ergonomic handle consistent with the popular Storz Ophthalmics reusable design. The forceps can be used for ILM, epiretinal membrane peel, macular puckers and cellophane maculopathy. The balanced, single-use design provides optimal feel and control for customers concerned about the fragile nature of reusable retinal instrument options, the company says.
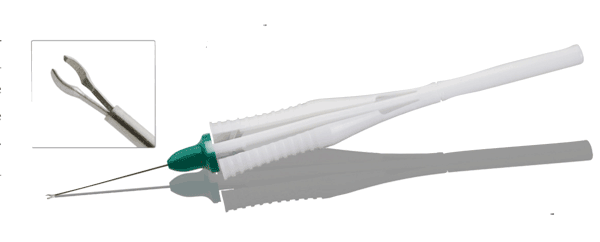
Both the 23- and 25-ga. versions are available in boxes of two and six for facility convenience. For information, visit storzeye.com/single-use-retina.aspx or call customer service at 1 (800) 338-2020.
Gulden Sizer Kits
Gulden Ophthalmics has in-troduced enucleation and evisceration Eye Orbit Sizer Kits that help surgeons and/or the surgical support staff rapidly and easily determine the proper size for orbital implants or other ocular prostheses.
In cases that require evisceration or enucleation, the Eye Orbit Sizer Kits save time and provide more accurate measurements and fitting of orbital implants, Gulden says. Each package contains four sterile, one-use, disposable sizers in 14-, 16-, 18- and 20-mm sizes.
The sizers are constructed of PMMA, providing excellent dimensional stability. The sizes are etched into the 3-inch-long handles.
Gulden also offers its Venus line of six sterile eye spheres from 12 to 22 mm, as well as non-sterile eye spheres and also a line of eye conformers. For information, call (215) 884-8105 or visit guldenophthalmics.com.
Akorn Gets Erythromicin Approval
Akorn announced that the Food and Drug Administration has granted approval of its Erythromy-cin Ophthalmic Ointment USP 1 g as a supplement to the company's already approved Abbreviated New Drug Application for Erythromycin Ophthalmic Ointment USP 3.5 g. The company has begun shipping the product to its customers.
Raj Rai, CEO of Akorn stated, "Recent market shortages have made this an attractive product which is complimentary to the other ophthalmic ointments Akorn currently sells.
This approval gives us access to both the sizes of Erythro-mycin Ophthalmic Ointment and positions us to compete effectively in all channels through which the product is sold." For information, visit akorn.com.
Pearls from the Deep
Edited by P. Dee G. Stephenson, MD, FACS, ABES, FSEE
Clearer Capsules, Better Vision With These Two New Instruments
With all the premium or lifestyle lenses we are implanting, hopefully we are constantly working on reproducible results for happier patients. That being said, I think we sometimes forget simple things like capsule polishing. My YAG rate has gone up somewhat because any striae or fibrosis on the capsule affects the quality of vision in these patients. So I have really been paying attention to this and have found two new innovative instruments that are really helping me.
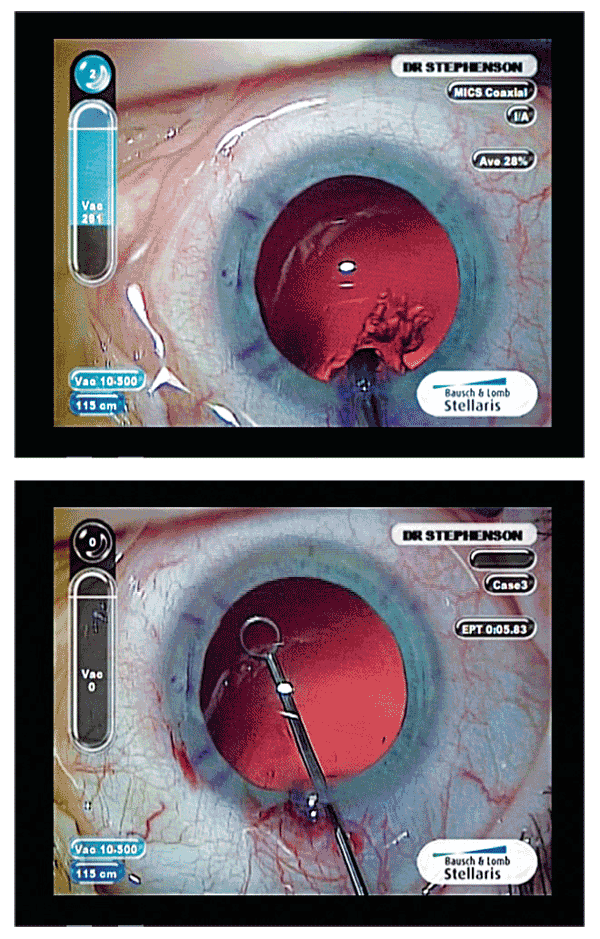
The first is the Silicone Capsule-Guard I&A (Storz), which you can lay right on the posterior capsule during your procedure and polish. The other new, innovative instrument is a modified (goes through a 1.8-mm incision) Whitman-Shepard capsule polisher. This instrument makes removing LECs from the inner surface of the anterior capsule and cleaning the equator of the capsular bag so much easier and safer. I find doing this in every case helps you to have clearer capsules for clearer vision.
The author has no financial interest in any product mentioned.



