Doctors hoping to diagnose and monitor glaucoma in their patients have a number of tools at their disposal, but visual field testing—which gives doctors a way to evaluate the functionality of the patient’s vision—is crucial. It certainly isn’t a perfect test, but it can provide information impossible to ascertain by other means.
“Visual field testing is subjective and has modest repeatability,” Gustavo De Moraes, MD, PhD, MPH, an associate professor of clinical ophthalmology at Columbia University Medical Center/New York Presbyterian Hospital in New York City, and chief medical officer at ORA Clinical, points out. “We want to minimize these issues as much as possible, although we can never completely eliminate them.”
Here, in the spirit of minimizing those factors, surgeons share their best advice for prepping patients; giving the test; deciding how often to test; choosing the best testing format for a given patient; evaluating the results; managing nonstandard patients; and combining visual field and OCT data.
Prepping Patients Ahead of Time
It helps to set patients and test-givers up for success before the patient sits down to take the test:
• Encourage new patients to practice for the test at home using visual field apps. “There are some new technologies that allow patients to do a visual field test at home,” notes Dr. De Moraes. “Those tests don’t provide as much useful information as the office-based test, but the patient gets a better understanding of how the test works. The result is that when they do the real test, they do it better. Sometimes these home tests are based around contrast—they may show a white background with a stimulus going from very dim to bright—but even those tests help patients be quicker and more reliable during testing.
“Besides the computer-based programs, portable devices, such as virtual reality tests, are now available,” he adds. “However, those involve loaning the patient an expensive piece of technology. The apps help to prepare the patient just as effectively.”
• Create a script your techs will use when describing the test. “It’s a good idea to have a set script that your technicians use when explaining the test to patients, because slight changes in their instructions can have a big effect on the test results,” notes Jo Ann Giaconi, MD, health sciences professor of ophthalmology at the Jules Stein Eye Institute, David Geffen School of Medicine, UCLA, and chief of ophthalmology at the Veteran’s Administration in Los Angeles. “For example, saying ‘Don’t press the button unless you’re certain you’ve seen a light,’ will produce very different results from saying, ‘Press the button if you think you’ve seen a light.’ Having a script to follow should eliminate this variable.” In fact, an inexplicable change in test results could reflect a change in test instructions.
“Different instructions have been shown to cause a difference in test results,” Dr. Giaconi points out. “If you don’t see another explanation for a change in your patient’s visual field, check to see if a different technician gave the test, or the technician changed their instructions for some reason.”
• Ask patients how they’re feeling before they take the test. “Unfortunately, many doctors don’t talk to their patients,” notes Dr. De Moraes. “I usually talk to the patient before the test and ask how they’re doing. I’d rather hear that they don’t feel well and postpone the test than end up with a questionable test result. I don’t want to make a treatment decision based on a poor test.”
Dr. De Moraes points out that a patient not feeling well often involves the patient being very tired. “A patient who’s tired during the test because they worked the night shift or just had a bad night’s sleep can result in lower sensitivity, even when the machine reports good reliability indices,” he says. “It may appear that the patient progressed when they were really just tired.
“Many times, a visual field with OK reliability indices will show a leap in progression, even though the patient’s eye pressure and visual fields have been fine,” he continues. “In that situation, I call the patient and ask how they were when they took the test. Almost always they’ll say something like, ‘Oh, I had a problem at home and I had a poor night’s sleep.’ If that’s the case, you should remove that test from your progression analysis.”
Prepping Patients at Test Time
These strategies will help ensure a useful test result:
• Don’t bring the patient straight from a brightly lit environment to do the test. “It’s important for the patient to have some time in the dark in the exam room,” Dr. De Moraes points out. “Let them get slightly dark-adapted before starting the test. Not doing this lowers the patient’s sensitivity to stimuli.”
• Make sure your techs explain the test before patients take it for the first time. “Your technicians need to educate new patients who’ve never done a visual field test before,” Dr. Giaconi points out. “They need to explain the purpose of the test and let the patient know what to expect. Sometimes it helps if the technician tests the visual field by confrontation, to help convey the concept of peripheral vision and make the point that the patient needs to keep their eyes fixated straight ahead during the test.”
“The doctor or technician needs to make clear how the test is done,” Dr. De Moraes agrees. “We need to remind the patient that sometimes the machine won’t show them anything. This is important to communicate, because patients can get anxious when they don’t see anything and start hitting the button, causing false positives. Remind the patient that this is not a test of skill; if they don’t see something, that’s fine.”
• Make sure your technicians are aware of potential language barriers. While this may not be an issue in some practice locations, it can be a problem in others. “Here in Miami, language barriers are frequently an issue,” says Swarup Swaminathan, MD, an assistant professor of clinical ophthalmology at the Bascom Palmer Eye Institute in Miami. “It’s important to make sure that the patient understands what to expect during the test. I’ve had several patients whose first language isn’t English; their OCT looks normal, yet their visual field is completely depressed. (See example, above.) Why do we see such a discrepancy? Turns out the patient didn’t understand the instructions. The technician needs to ensure that the patient understands how the test works and what’s expected of him or her, especially when the patient is a first-time test taker.”
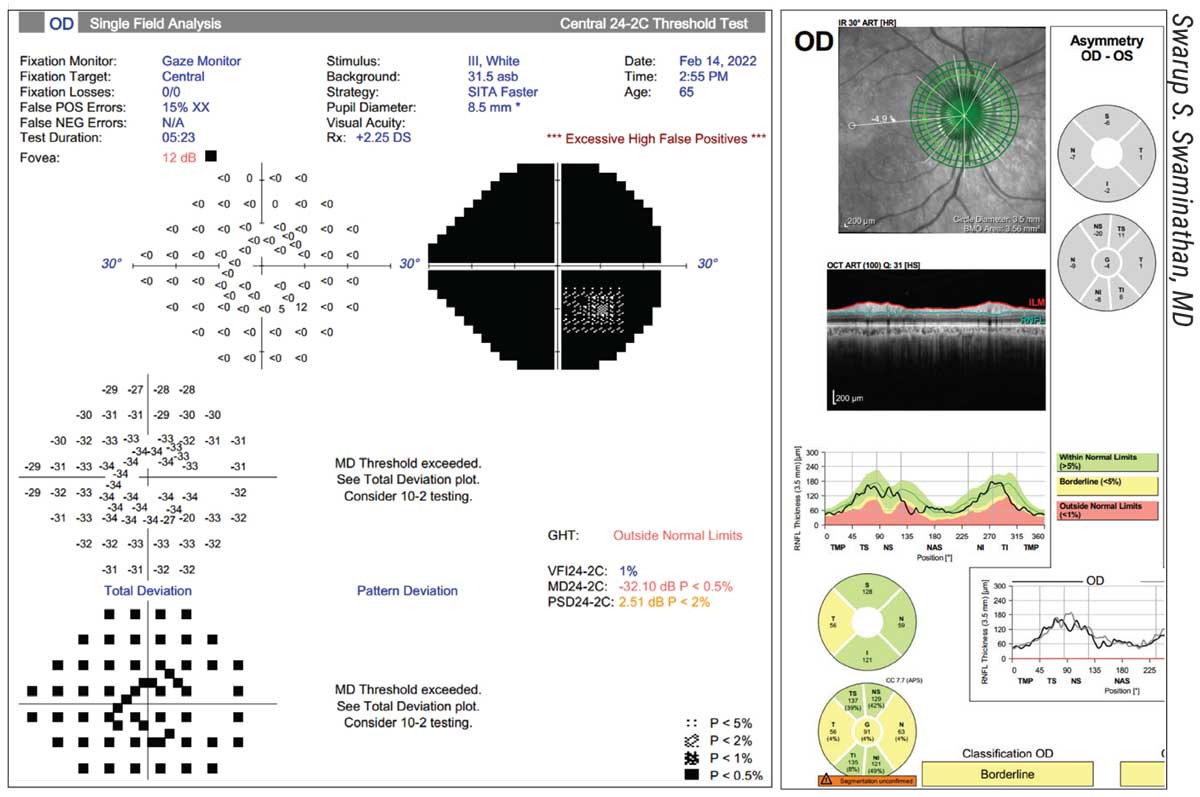 |
| In some areas, a language barrier can explain a poor result. Above: The right eye of a Haitian Creole-speaking patient. The RNFLT is robust, yet the visual field is completely depressed. The instructions were given in English, which the patient didn’t understand. |
• Make sure your technicians are on the lookout for potential eyelid problems. “Technicians need to be aware of issues such as dermatochalasis or ptosis, which are common in our elderly patient population,” says Dr. Swaminathan. “These conditions can affect the visual field and confound the results. Taping up the eyelid can be very helpful in such situations.”
• Have the technician make sure the patient is comfortable. “The test takes a few minutes, and patients get tired,” says Dr. Giaconi. “If they’re not sitting in a comfortable position, that will affect the results.”
• If the patient has dry eyes, put in a drop of artificial tears before testing. “Dry eyes are common among glaucoma patients,” notes Dr. De Moraes. “In that situation I strongly recommend using an artificial tear before testing. That will help the patient’s vision be more stable during the test.”
• Tell patients it’s perfectly OK to blink during the test. “Patients often assume they can’t blink during the test,” Dr. De Moraes points out. “That’s not true at all. They should blink during the test, because if they don’t their vision will get blurry. The machine detects when the patient is blinking and takes that into account, so you should advise your patients to blink as much as they want.”
• Tell the patient that it’s OK to ask to restart the test. “Patients often know that they missed something, for example because they know they weren’t paying attention at the beginning of the test,” Dr. De Moraes says. “They should be told that it’s OK to ask to start the test over. Furthermore, they should be told that they can pause the test, too. In many cases, just pausing for a few seconds will provide a reliable test result, which you wouldn’t otherwise get. Pausing or restarting the test may obviate the need for a two-hour office visit a couple of months later.”
• Make sure the lens is appropriately placed. “Occasionally when looking at a test result you’ll find all of the outer points on the visual field have a decibel value less than zero, while all the points just adjacent to it are normal,” says Dr. Swaminathan. “The idea that glaucoma would only affect the outermost periphery of the visual field but not the adjacent areas is highly unlikely. This finding is most likely caused by the rim of the lens that was placed in front of the patient’s eye. The technician should ensure that the lens is appropriately placed and not blocking the patient’s field of view.”
• Don’t turn off the gaze-tracking software when testing. “There’s a gaze-tracking device in the machine—basically an infrared light in the machine that watches your pupil to see when your eye moves,” Dr. De Moraes explains. “It flags a problem when the eye is moving too much, and may ask you to restart the test. Unfortunately, in busy clinics, technicians sometimes turn that off so they can do the test more quickly without having to restart if there’s a problem.
“Of course, there are other tests for reliability, so if this is turned off, you’re not necessarily losing the value of the test,” he adds. “On the other hand, it provides an important piece of information about whether the test was good or not. In my experience, turning the gaze tracker off to save time is a big mistake.”
Pearls for Giving the Test
Once the test starts, the person giving the test needs to remain watchful.
• Make sure your technicians actively monitor the patient’s test performance and respond if there’s a problem. Dr. Giaconi points out that the technician can’t just start the test and then go text a friend. “They need to actively monitor the patient,” she says. “If they see the patient isn’t doing something correctly, they can stop the test, re-educate, re-instruct and restart the test. For example, on the monitor the tech can tell if the patient isn’t holding fixation on the center target. They can see if the patient isn’t responding at all, or is responding too quickly, which suggests the patient is anxious about the test.”
She notes that it’s important for the technician to watch the fixation monitor during the test. “If it’s clear that the patient’s eye was closed for half the test, or they had horrible fixation throughout the test, that test should be repeated,” she says. “If the tech is paying attention, they can try to coach the patient to do better, although some patients don’t do well no matter what you do to help.”
“The technician should be available to ensure that the patient is taking the test correctly,” Dr. Swaminathan agrees. “If the patient isn’t maintaining fixation, for example, the technician should remind the patient to do so. It might be helpful for the technician to point out that the doctor could ask the patient to return sooner than usual to repeat the test if it’s found to be unreliable.”
• Make sure the patient sees the first stimuli shown at the beginning of the test. “This is important because today’s machines have an algorithm that starts with a very dim stimulus,” Dr. De Moraes explains. “That dim stimulus defines the threshold of brightness used throughout the rest of the test. So at the beginning the technician should assist the patient and make sure that what they think they see is real.”
• Advise technicians how to proceed if the patient doesn’t appear to be responding. “Sometimes in very advanced glaucoma, the technician may see that the patient isn’t responding,” says Dr. Giaconi. “This could indicate that the stimulus size is too small and they need to use a different stimulus size or test strategy. For example, if you’re testing with the 24-2 test and the patient has a very small island of vision and isn’t seeing anything, there’s not much point to making them sit there for eight minutes. It’s better to switch to a 10-2 testing strategy.
Setting a New BaselineSurgeons point out that one of the most common errors made by doctors comparing a series of tests to look for signs of disease progression is forgetting to set a new baseline after an intervention had occurred. “Any intervention, such as glaucoma surgery or laser trabeculoplasty, will alter the rate of change in visual field testing for a particular eye,” says Swarup Swaminathan, MD, an assistant professor of clinical ophthalmology at the Bascom Palmer Eye Institute in Miami. “It’s important to make note of any interventions that have been done and adjust your interpretation accordingly by setting a new baseline. In many cases, the doctor is provided with a printout of all prior visual fields. The physician needs to carefully identify when an intervention has occurred and review the visual field data in this context. This can have significant implications for how the patient is managed.” The same rule applies when the type of visual field test the patient is taking is changed, which is one reason surgeons may hesitate to change testing formats in a progressing patient. So be sure to account for any change in strategy if you’re looking for progression over a series of tests. |
“An experienced technician might be able to make that call,” she adds, “but in any case, it’s important to remember that changing the strategy will interfere with the surgeon’s ability to use previous tests for monitoring progression. That means the doctor has to be consulted before making such a change.”
How Often Should We Test?
“This decision is always individualized,” Dr. Giaconi explains. “If you have a glaucoma suspect at risk of developing glaucoma whose testing continues to be normal, you might do a visual field test once a year. But if you’re seeing someone for the first time who has established visual field defects, then you want to figure out how quickly things are changing. In that situation you should do visual fields two or three times a year to get a sense of the rate of change.”
What if the patient is a novice with no previous data? “If the first visual field is normal and all other exam findings point to a low risk for glaucoma, I’d wait a while before retesting the patient,” says Dr. Giaconi. “If the first test result is abnormal—for example, if the patient has just a small island of vision left on the standard 24-2 test—then you might decide to have the patient come back in a month or two to map out the central field.
“We don’t do the two tests in the same day, although some practices do,” she adds. “In theory, there’s no reason you can’t. It depends on multiple factors, such as how tired the patient is from taking the first visual field test, and more mundane factors such as when their ride is coming to pick them up.”
Dr. Giaconi points out that sometimes you may want to retest fairly quickly because of an unexplained result. “For example, I saw a patient this week who was coming back to repeat her right eye visual field because it looked suspicious for change at the previous visit. This week’s field was very depressed and much worse than the last test. I was concerned that she had developed a neurological problem. We retested both eyes immediately that day, and fortunately, the second time around the right eye tested normal. I think she may not have been paying close attention during the earlier testing.”
• Expect a learning curve in many new test-takers. “It can take patients a few tests to really understand what we need them to do, so learning curve artifacts are common,” Dr. Giaconi notes. “In a few patients, learning curve errors can continue through more than a half-dozen tests.”
Combining Visual Fields & OCT
Visual field testing and OCT are both essential for glaucoma diagnosis and following progression. In particular, surgeons find them useful for confirmation of findings.
“Comparing your visual field data to OCT data can be very useful, especially with early glaucoma,” says Dr. Giaconi. “When there’s just a faint visual field defect, it’s nice to see correlation on OCT. Sometimes the OCT may show that the area in question is still in the green zone, but you can see that the measurement is lower than in the other eye. So you may find supporting evidence for your concerns.”
Dr. Swaminathan says he likes to use OCT data as confirmation for what some visual fields show, and vice versa. “I tend to alternate between these test modalities,” he says. “In early glaucoma, you’re more likely to identify OCT changes with minimal visual field changes, so the utility of comparing them is limited. However, once you observe a moderate amount of disease, there’s some degree of linear relationship between OCT RNFL thickness and visual field mean deviation or visual field index.
“So, if an OCT looks a little suspicious, I check the visual field, or vice versa,” he continues. “I use either test to confirm or refute suspicions of progression raised by the other modality. Of course, the studies must be of reasonable quality and relatively free of artifacts to incorporate them into this type of analysis.
“Since I alternate tests, I usually obtain at least one visual field and one OCT per year,” he adds. “This can support a reasonable trend analysis over a few years.”
Dr. De Moraes advises never looking at either data source in isolation. “Avoid basing your decisions on just one of them,” he says. “Once you have both, it’s important to do a topographical comparison of corresponding regions. A visual field is very subjective; the patient can be tired, or have other eye diseases or a neurological issue. If you see that the OCT abnormal region matches the abnormal area in the visual field, it provides some confirmation that the defect is real and was more likely caused by glaucoma.”
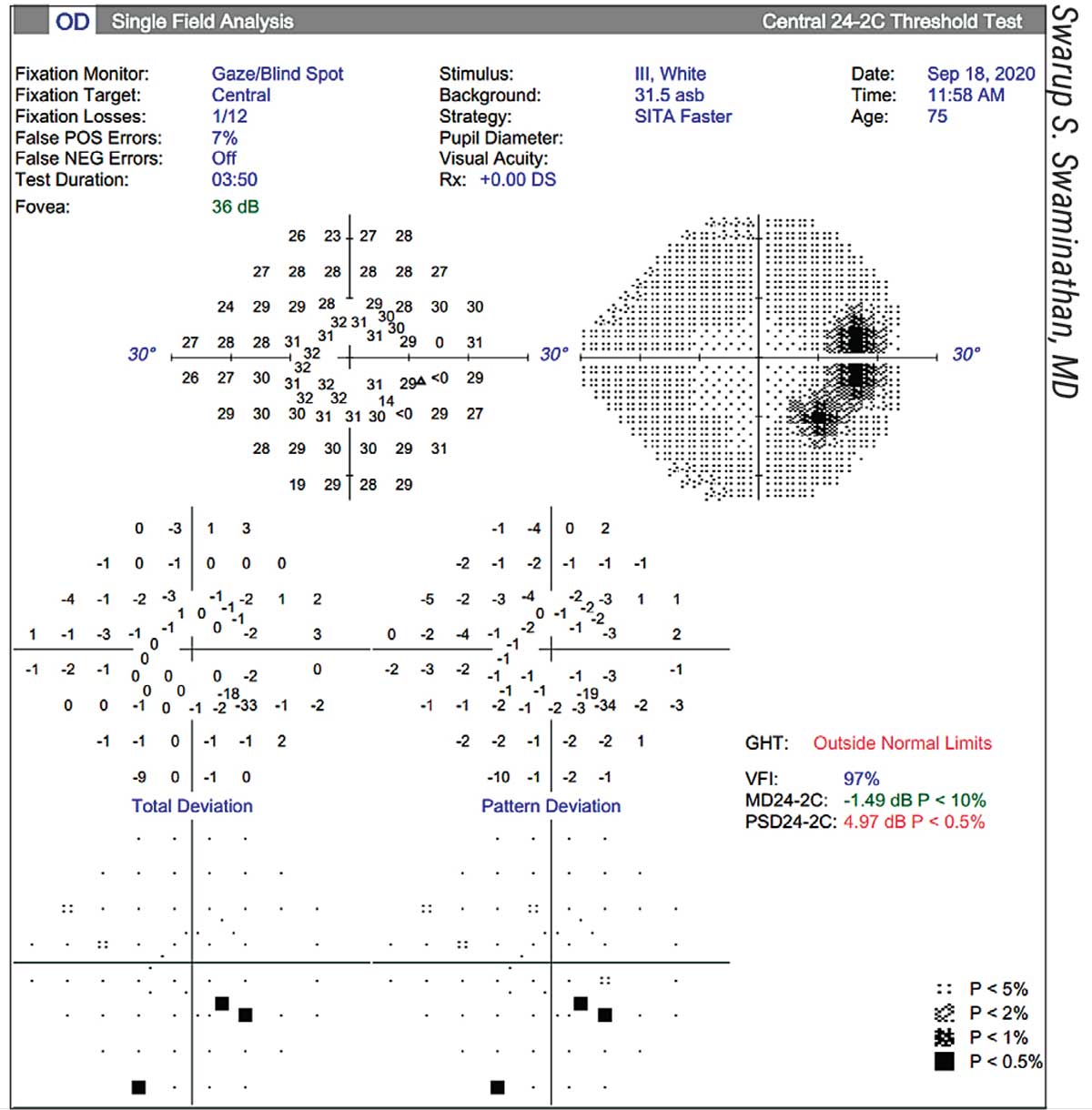 |
| This patient has what appears to be an arcuate defect, causing the Glaucoma Hemifield Test to be “outside normal limits.” However, the patient actually has a large chorioretinal scar adjacent to the optic nerve. |
Advanced Glaucoma
“In advanced glaucoma, visual fields become highly variable,” Dr. De Moraes points out. “It’s a subjective test, and as the disease progresses, the repeatability of the test declines. If you test an advanced patient two days in a row, the tests could look very different. That’s problematic because in advanced glaucoma, any loss is a big deal for the patient.” These strategies can help:
— Try switching to the 10-2 test. “We know that the central part of the visual field tends to be the last to go away in glaucoma,” says Dr. De Moraes. “The 10-2 test focuses on the central region.”
“In advanced glaucoma cases, the 10-2 test is the way to go,” Dr. Swaminathan agrees. “Remember that in advanced glaucoma, OCT has limited utility. There are some aspects of ganglion cell loss that can be followed in advanced disease, but it’s harder. So, the visual field becomes your best friend.”
Dr. Giaconi concurs. “If the patient’s glaucoma is so advanced that the 24-2 no longer shows a pattern deviation plot, that usually means the patient is down to a central island, so we’ll switch to a 10-2 strategy,” she says.
— Try switching from a Goldmann size III stimulus to size V. “When a patient reaches the floor effect with size III, you may be able to follow them longer by switching to size V,” notes Dr. De Moraes. “This will improve the repeatability of the test.”
— Do more frequent testing. “Because of the higher variability of visual field test results from advanced glaucoma, the signal-to-noise relationship shifts toward noise,” Dr. De Moraes points out. “One way to increase the signal strength is to do more tests. In early glaucoma you might test the patient once or twice a year; in advanced glaucoma, you may need to test patients three or four times a year.”
“There’s one good thing about patients with advanced disease,” Dr. Swaminathan adds. “Many of them have been doing visual fields for a long time, so they’re excellent visual field takers. As a result, test reliability concerns are a nonissue with these patients, although you may see a high false negative rate due to severe disease.”
When to Discard a Result
Given the subjective nature of visual field testing, it’s important to watch for indications that a test result has been altered by something other than a change in the patient’s field:
• Keep an eye on the false positive rate. “Among the reliability indices, the one that’s widely considered the worst to have a high score on is the false positive score,” says Dr. De Moraes. “A false positive means that the patient was responding as if seeing a stimulus when a stimulus wasn’t shown, or when the stimulus was too dim for the patient to actually see.
“Doctors often call these patients ‘trigger-happy,’ ” he continues. “These patients are often anxious; they think they’re fighting with the machine. Sometimes they’re video game players. The problem is, if you have a glaucomatous defect, it may not show up because the patient is pressing the button all the time. The results may even suggest that the patient improved since the previous visit, which rarely happens in glaucoma. So false positives are particularly detrimental to finding progression.”
“If false positives are terribly high, we’d scrap that test result, because usually in that situation the result looks a lot better than it really is,” says Dr. Giaconi. “Some patients repeatedly produce false positives that aren’t related to the disease, sometimes because of anxiety. A high false positive rate is problematic because the ‘trigger-happy’ patient’s excess responses throw off the machine’s calculations. For example, points with normal sensitivities will now end up appearing depressed. These test results can be very unreliable.”
• On the other hand, consider other factors before automatically discarding a test due to high false positives. Dr. Swaminathan points out that there’s some debate regarding what constitutes an unusable test. “The literature on this topic is changing,” he says. “It’s becoming clear that fixation losses don’t necessarily have a significant impact on the visual field result. False negatives are often elevated in patients with advanced glaucoma. With respect to false positives, conventional wisdom suggests that a rate greater than 15 percent is deleterious to the quality of a visual field. However, a test can have a high false positive rate yet remain somewhat useful, so this isn’t an across-the-board reason to reject a test.
“I’ve seen tests with a false positive rate of 17 percent, where the test was fairly consistent with past visual fields,” he notes. “In that case I’ve incorporated the test into the series. Many physicians will use 15 percent as their cutoff, but others may draw a different line in the sand—especially if the test is consistent with previous tests. The jury is still out on this issue.
“Of course,” he adds, “if the pattern deviation plot has defects but the total deviation plot is normal (referred to as the ‘inverted cataract’ pattern), or the glaucoma hemifield test has a result of “abnormally high sensitivity,” the test is unlikely to be of value.”
In terms of how seriously doctors should take the three reliability indices—fixation loss, false negatives and false positives—Dr. De Moraes agrees there’s some debate. “Some doctors believe that fixation loss and false negatives may not be as important as we used to think,” he says. “It’s true that they don’t seem to impact progression analysis very much. However, most doctors agree that a false positive score above 15 or 20 percent is bad.”
• Look for significant changes in reliability indices from one test to the next. “These machines have three main reliability measures: fixation losses; false negatives; and false positives,” Dr. De Moraes notes. “If one of them changes dramatically from one test to next, you need to proceed cautiously. It’s perfectly OK for patients to not have perfect reliability indices—no one does. But consistency between tests is important. If one test has a false negative response of 20 percent, and the next is 22 percent, that’s OK. But if the next time it’s 40 percent, that may not be OK. Consistency between tests suggests more reliability.”
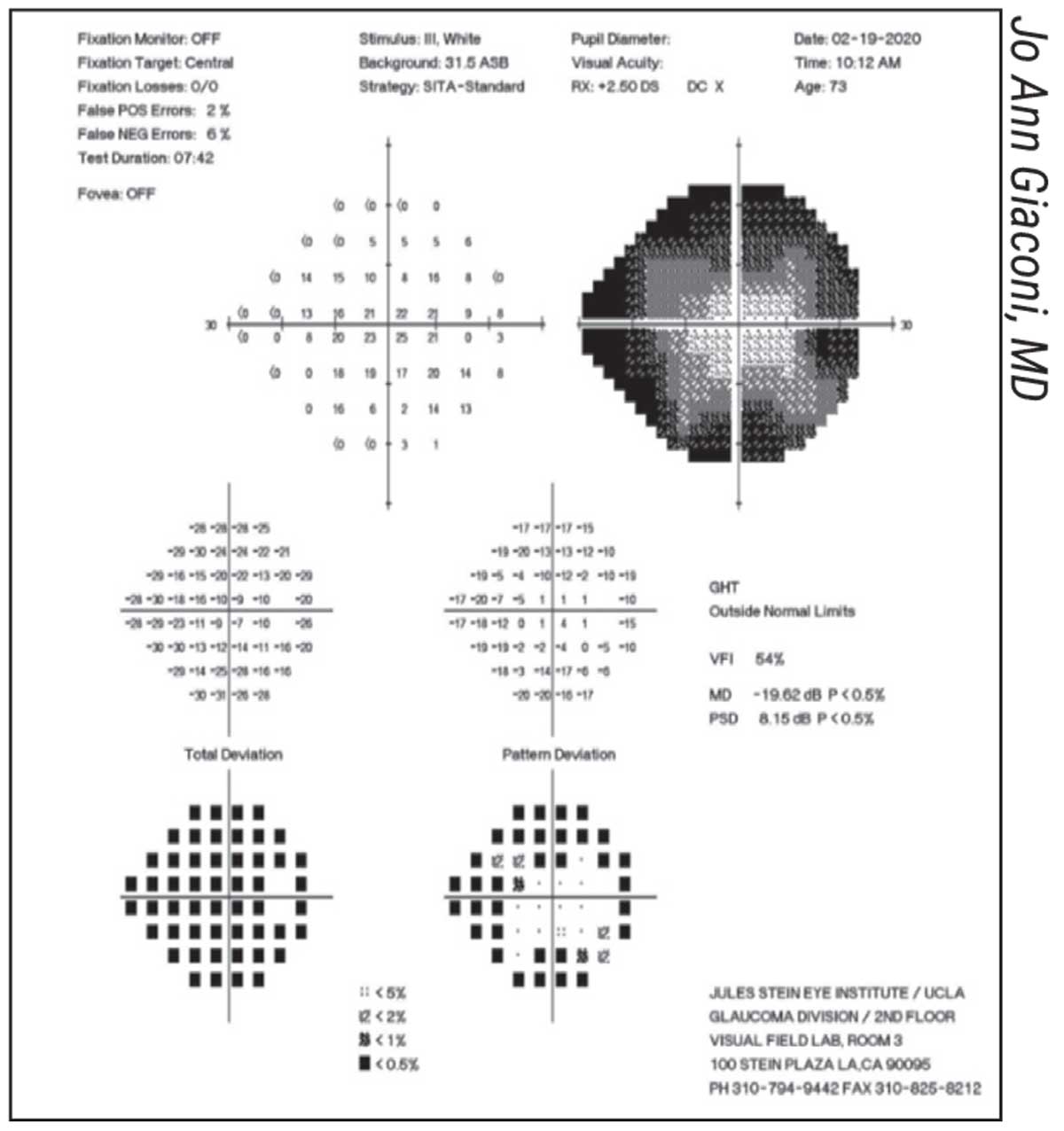 |
|
A classic “cloverleaf” pattern artifact, suggesting the patient became sleepy or distracted after the beginning of the test. |
Common Testing Artifacts
It’s easy to be fooled by artifacts that create the illusion of a problem where none exists (at least in terms of glaucoma). Surgeons note these artifacts as being especially common:
• The “cloverleaf” pattern. Dr. Giaconi points out that this artifact—in which the central points in each quadrant are much lighter than the surrounding points—is a common indication that a test is unreliable. “The computer has four primary points that it tests first, near the center of each quadrant,” she explains. “Often the technician walks away to set up another patient, and that’s when the patient starts falling asleep or being distracted. The result is a pattern resembling a four-leaf clover.” (See example, above.)
“If you believe this pattern is an artifact, examining the optic nerve may confirm that it is,” she adds. “It will look much healthier than a nerve that would actually cause such a poor visual field.”
• Lens-rim artifacts. As noted earlier, you may occasionally find all of the outer points on the visual field have a decibel value less than zero, while all the points adjacent to it are normal. As Dr. Swaminathan noted, this finding is most likely caused by the rim of the lens that was placed in front of the patient’s eye.
• Bogus scotomas. A couple of conditions can create the appearance of a scotoma where none actually exists:
— A tilted disc in a high myope can create the appearance of an arcuate scotoma. “Most nerves in this situation are tilted inferiorly, so the scotoma usually appears to be superior,” notes Dr. Giaconi. “In a busy clinic, you may encounter this as often as once a week. Your physical exam should be able to confirm the true nature of the situation.”
— False positives can be associated with “white scotomas.” “A true scotoma in a visual field looks black,” says Dr. De Moraes. “If a scotoma is white, that indicates that instead of visual loss, the vision in that area has improved. That’s caused by a trigger-happy patient. If you have too many false positives, you’ll start to see islands of improvement that aren’t real.”
When is the 24-2C test most appropriate? Among the format options open to doctors testing a patient’s visual field are three different testing strategies. The recent addition of the third testing strategy, the 24-2C, has raised questions about when doctors might want to switch to using it.“The [standard] 24-2 tests 54 points in a 24-degree radius of vision,” Gustavo De Moraes, MD, PhD, MPH, an associate professor of clinical ophthalmology at Columbia University Medical Center/New York Presbyterian Hospital in New York City, explains. “The 10-2 test covers 68 points in the central 10 degrees—the macula. So instead of 54 points spread over a broader area, with only 12 points in the central 10 degrees, the 10-2 packs 68 points into that much smaller area. “One way to check both the periphery and the macula would be to alternate between these two strategies,” he continues. “However, because you can’t directly compare the data from different strategies, alternating between the tests at different visits would cut the number you can compare in half—which is not good for monitoring progression. “The 24-2C strategy is designed to help with that problem by adding an additional 10 points to the central region of the 24-2 test, for a new total of 22 points in the center,” he explains. “As a result, your ability to detect field loss in the macula increases. The test doesn’t take any longer than the 24-2 test, because the manufacturer uses a slightly faster algorithm. The argument in favor of using the 24-2C is that you’re still able to test your patient’s peripheral vision—which you can’t do with the 10-2—but you get increased ability to detect central loss. Glaucoma sometimes starts in the periphery, so it’s good to still be able to test that; but now we can also test the central region with more accuracy. We don’t need to switch back and forth between tests. “As a result, many doctors have transitioned to using the 24-2C for all patients,” he says. “Of course, in some cases you might still need to eventually switch to the 10-2 test as a patient’s vision deteriorates. The 22 central points tested in the 24-2C don’t come close to the 68 points tested by the 10-2. The good thing is that you may not need to make that switch as often before the disease become very severe.” Why not just switch to the 24-2C as your primary test going forward? “One argument against doing this is that you lose a little bit of accuracy because the testing is done faster,” Dr. De Moraes points out. “The other problem is that every time you switch to a different test you have to reset your baseline. If you have a practice with many patients you’ve been following for many years, switching to the 24-2C could decrease the usefulness of that older information. For that reason, many doctors are switching to the 24-2C for new patients, but sticking with the standard 24-2 for long-time patients. “If the manufacturer chose to release the 24-2C with the conventional algorithm used in the standard 24-2, I imagine a lot of docs would be more inclined to use it across the board, because then practices could continue to include the old data in their progression analysis,” he adds. “The downside is that the companies would have to modify their software and databases. That’s not a small thing.” —CK |
Choosing the Right Test
“We have an armamentarium of different visual field tests and parameters to work with,” Dr. Swaminathan notes. “For example, we can decide the number and location of points to test by choosing between the 24-2, 10-2, and 24-2C test patterns. The 24-2C adds some central points to the 24-2 test, making it possible to catch some paracentral changes you might otherwise miss. I often use the 24-2C as my first choice for new patients, except in cases of advanced glaucoma, in which using the 10-2 test pattern is more helpful.
“We can also choose the testing strategy—SITA Standard, Fast or Faster,” he continues. “SITA-Faster isn’t quite as thorough, but it can be beneficial in patients who have difficulty maintaining attention, so for many patients I’ll use it as the default test. Of course, if a patient has been followed with SITA Standard for years I’ll make an exception, but in general, for straightforward cases, I favor it because it’s quicker, easier for the patient and helps with clinic flow.
“Lastly, we can choose the stimulus size—size III or size V,” he points out. “Some patients aren’t good field takers, but instead of giving up on them and only following them with OCT, it might be worth trying a size V stimulus. Typically, we’d reserve size V for patients with an acuity of 20/80 or less, but I have some patients who have excellent visual acuity, yet are poor test-takers; they do much better with a size V stimulus. I tend to follow these patients using that stimulus size.
“Visual fields are one of the few subjective tests in ophthalmology,” he notes. “We have to take a few things into account: Is the patient sleepy? Did the patient understand the instructions? Will the severity of the disease affect the test accuracy? It’s important to identify what combination of test parameters will work best for a given patient. Often, you’ll want to stick with whatever test the patient’s been using so you don’t have to reset the baseline for following progression, or because you’re watching a nuanced detail that a given test has been capturing. But if I’m seeing a new patient or someone who’s a glaucoma suspect, I tend to use the SITA-Faster strategy with the 24-2C pattern.”
Two key strategies to remember:
• Be consistent about which testing algorithm you use with a given patient. “The visual field machine has different algorithms—how fast the test is done, time between test points, and so forth,” says Dr. De Moraes. “I often see patients from other clinics being tested with different algorithms at different visits. The problem is, the results aren’t comparable; you can’t determine whether the disease has progressed if you use a different algorithm every time you test the patient. So I recommend being consistent.
“Using a faster test always comes at the expense of not testing each point as thoroughly,” he continues. “Instead of testing a point five times, it might only test it twice, so you lose a little bit of accuracy. However, many practices prefer to use the fast tests for every patient, and it’s not wrong to do that. Consistency is the key. As long as you use the same test every time, that’s fine. Just don’t switch tests on different visits.
“There’s no one ‘perfect’ algorithm,” he adds. “The science doesn’t support the idea that one is always better than the others. So when the technician brings the patient to take the test they should look at the previous printout to see what algorithm was used. At times, the doctor may want to change the algorithm in order to better follow the patient. However, the algorithm is often changed by the technician, by mistake.”
• Don’t be shy about using the 10-2 testing strategy. “The 10-2 focuses on our central vision,” Dr. De Moraes notes. “We use our central vision for most important functions, so the condition of our central vision has implications for quality of life. Also, combining an OCT of the macula with a 10-2 test will help find correlations regarding any defects you uncover.”
Non-standard Patients
Some patients have special circumstances that are worth taking into account:
• If the patient has had radial keratotomy, ask the patient at what time of day their vision is clearest. “The main problem with an RK patient is that their vision can fluctuate from morning to night,” Dr. Giaconi points out. “The patient may have a preferred time of day when they feel their vision is clearest. Ideally, that’s when you should test them.”
• If the patient has a central scotoma, try using an alternate fixation point. “If a patient has macular degeneration with a central scotoma, they won’t be able to see the central fixation target because it’ll be inside the scotoma,” notes Dr. Giaconi. “However, there are lights in the shape of a diamond that you can light up in the bowl below the central fixation LED. You can ask the patient to use those as a fixation point by keeping them in the same place in their visual field. This gives them an eccentric target to fixate on.”
• If the patient has a multifocal lens implant, ask whether a near add lens is needed. While surgeons agree that they’d hesitate to place a multifocal IOL in a patient with glaucoma, the disease can occur after an individual already has such an implant.
Dr. Swaminathan says he generally doesn’t do anything different if a patient has a multifocal IOL implant. “If they have a true trifocal lens, it’s possible they may not need a lens placed in front of the eye for visual field testing,” he says. “If the patient is truly spectacle-independent—if they say they never wear reading glasses—then they shouldn’t need a near add, and the technician should be aware of that. But unless that’s the case, I wouldn’t do anything different.”
“In theory you don’t need to put in a trial lens if the patient taking the visual field test has a multifocal implant,” says Dr. Giaconi. “However, we’ve found that even patients in this situation usually find it easier to take the visual field test with the plus add in front of their eye.”
“A multifocal lens can cause some loss of sensitivity, similar to that seen with a cataract,” says Dr. De Moraes. “It can cause a diffuse loss, making it seem like the patient has worse glaucoma. So, make a note when a patient has a multifocal implant. And of course, if the implant was done during the time you’ve been caring for the patient, reset your baseline after that point.
“Generally, putting a lens in front of the eye is necessary in any case where the patient isn’t 20/20 or J1 in near vision,” he continues. “Regardless of the lens in the patient’s eye, you should always add a lens that gives the patient the best corrected near vision for the test. Either way, a little diffuse vision loss on the test shouldn’t be confused with glaucoma.”
• If a patient is bad at taking visual fields, don’t give up too quickly. “With some patients whose visual field results are unusable you can simply rely more on OCT scans—assuming OCT is still giving you good information,” Dr. Giaconi suggests. “For other patients, you’ll have to rely mostly on your examination. I recently had a patient who absolutely refused to do a visual field test. We explained to him how much it helps us determine if his glaucoma is getting worse, but he said, ‘No, it hurts my neck. I’m never getting into that machine again.’ So we’re stuck managing his glaucoma without a visual field, which is not ideal.”
Dr. Giaconi notes that you shouldn’t give up on a patient’s test-taking skills too quickly. “Sometimes, when a patient isn’t a good test performer in general, we’ll switch to a size V stimulus to see if they do better,” she says. “Often that helps us get usable data.”
The Impact of Health Problems
“It’s important to ensure that any abnormalities you’re seeing are really the result of a progressive optic neuropathy due to glaucoma before altering your management,” Dr. Swaminathan points out. “A myriad of conditions can affect the quality of this test; a visual field accounts for everything in the visual axis. The test result can be affected by eyelid problems such as ptosis or dermatochalasis, dense cataracts, severe intraocular inflammation, chorioretinal scars, diabetic retinopathy, macular edema, retinal detachment, epiretinal membranes, pituitary adenomas and optic neuritis. The visual field test isn’t just specific to glaucomatous damage.
“I’ve seen many patients who were diagnosed as having glaucoma because their visual fields were abnormal,” he continues. “Some of these patients had severe proliferative diabetic retinopathy; they were getting injections every month, and their visual fields had a nondescript pattern that wasn’t typical of a glaucomatous scotoma. A few years ago, I met a patient who was referred for trabeculectomy because the referring doctor thought that his IOP needed to be 8 or 9 mmHg. He had a large chorioretinal scar along the superior retinal arcade that corresponded to his visual field defect. His scotoma was nonprogressive, so we took him off most of his topical IOP-lowering medications. On a nightly prostaglandin analogue, his IOP has remained in the high teens. He feels a lot better due to the reduction in drop burden, and his visual field has remained stable.
“It’s essential to always consider other possible explanations for the visual field result,” he concludes. “If the visual field doesn’t have a classic glaucomatous arcuate pattern or paracentral scotomas suggestive of normal tension glaucoma, it would be wise to ask whether the patient truly has a progressive glaucomatous optic neuropathy. A mean deviation value of -12 dB doesn’t necessarily mean the patient has glaucoma. We have to treat the patient, not the visual field.”
In terms of other health problems that can affect visual field test results, surgeons note these in particular:
— Cataract. “Sometimes a patient has an early cataract overlying a glaucoma defect,” Dr. De Moraes points out. “The cataract causes the appearance of a more diffuse loss, making your evaluation more difficult. Glaucoma typically causes a more local loss in the early stages; later it becomes more diffuse. In someone whose cataract is progressing, the visual field will be diffusely impacted, giving the impression of glaucomatous defects. So anytime you see a more diffuse loss, do a dilated slit lamp exam to see if the cataract got worse. Whenever I see diffuse defects becoming worse from test to test, I take a look. Usually this happens slowly, but sometimes it can get significantly worse from one visual field to the next.”
— Retinal issues. “Retina diseases such as macular degeneration can cause visual field loss that can be mistaken for glaucoma,” Dr. De Moraes points out. “If there’s any reason to suspect this, bring the patient back to the exam room; take a look at the retina, and do an OCT if possible, to rule out retinal conditions. How much of an impact a retinal problem has will depend on the patient’s condition. Very bad retinal disease can make the visual field useless, but that may not be the case for the vast majority of patients who have early disease, such as epiretinal membranes of early dry macular degeneration. Just keep in mind that some of the defects you see may not be from glaucoma.
“This is another reason to always check both the OCT and visual field data,” he notes. “If I look at the eye and see drusen from macular degeneration, and the patient has a central visual field loss but the OCT doesn’t show any nerve fiber layer loss in that region, I assume it’s caused by macular degeneration—not by the glaucoma.”
— Neurological conditions. “If the patient has had a stroke, that can cause visual field defects too,” says Dr. De Moraes. “This is another reason it’s important to keep talking to the patient. You should always ask, since you were here six months ago, did anything unusual happen in your life? They may say, ‘Well, I fainted’; or ‘I had a seizure’; or, ‘I was hospitalized.’ A stroke or a tumor could cause visual field loss that will make it harder to diagnose glaucoma or monitor progression.”
“Any visual field result with a scotoma that respects the vertical midline is concerning for a neurologic condition,” note Dr. Swaminathan. “The patient should be evaluated by a neuro-ophthalmologist promptly. Intracranial tumors, strokes and pituitary tumors all can manifest on the visual field.”
— Other systemic problems. “A patient with Parkinson’s or Alzheimer’s could have trouble pushing the button,” Dr. De Moraes notes. “Alzheimer’s could cause the patient to have trouble following instructions. The point is that you need to look at the patient holistically. Make sure you see the big picture before evaluating a test result.”
— Medications the patient is taking. “Some medications can affect performance on the visual field, particularly those that cause somnolence,” Dr. De Moraes points out. “I have a patient who started taking a new drug for anxiety that caused some central nervous system depression. I could clearly see a degradation of the visual field once the medication was started. It wasn’t that the glaucoma got worse; the patient was just having a harder time taking the test. So every time you see a patient, review their medications and ask if any new medications have been started.”
Still the Gold Standard
“Even though OCT has many advantages compared to visual fields—it’s objective, automated and more repeatable—visual fields are still our gold standard for assessing visual function,” Dr. De Moraes points out. “For clinical decisions—even for clinical trial purposes—it’s the visual field that matters. The FDA currently only accepts visual field endpoints in trials for new glaucoma drugs aimed at slowing progression. Despite its limitations, a visual field is currently the best test to correlate with the patient’s quality of life and quality of vision, especially when we’re monitoring progression.”
“A visual field may not be the ideal test, but sometimes it’s the best data we’ve got,” Dr. Swaminathan agrees. “Learning what works best for the patient is essential. At the end of the day, the goal is to obtain useful data that one can use to monitor the patient’s disease.”
Dr. De Moraes is a consultant for Carl Zeiss and has received research support from Heidelberg Engineering. Drs. Giaconi and Swaminathan report no financial disclosures relevant to any topics discussed in the article.