The prevalence of both glaucoma and ocular surface disease, or OSD, is increasing as the population ages. It has been estimated that as many as 20 percent of Americans have some dry-eye symptoms. The prevalence of dry eye increases with age and is most common among postmenopausal women.1,2 Often, the presence of one of these disease states contributes to the symptoms or complications of the other. Glaucoma is a frequent complication in patients with severe OSD.3 In fact, the overall prevalence of glaucoma in these patients is estimated in some studies to be as high as 75 percent.3 Furthermore, many treatment modalities for OSD, such as topical corticosteroids, contribute to the risk of glaucoma or can exacerbate preexisting glaucoma.3
Chronic use of preservative-containing topical ophthalmic products for the treatment of glaucoma can often contribute to the development or worsening of OSD. Many patients with glaucoma have some degree of ocular surface damage. It is estimated that dry eye may be present in approximately 40 percent of glaucoma patients.4,5 Glaucoma treatment typically involves the use of one or more topical ophthalmic medications for the reduction of intraocular pressure. These medications must be used chronically, resulting in high cumulative exposure to potentially toxic preservatives contained in the product formulation.6 Research demonstrates that significant exposure to glaucoma medications preserved with benzalkonium chloride (BAK) can produce alterations in tear film function and inflammatory conjunctival infiltrates.7
This article will examine the presence of OSD in the glaucoma patient, the role of preservatives in glaucoma medications that are chronically used in these individuals, and the alternative solutions to consider in this setting.
OSD in Glaucoma Patients
A common complication of OSD is dry eye, which is often caused by tear instability and hyperosmolarity that create a proinflammatory condition. In fact, recent evidence indicates that a hyperosmolar environment is itself proinflammatory.8 Although the causes of dry eye are myriad, they tend to share several common characteristics.
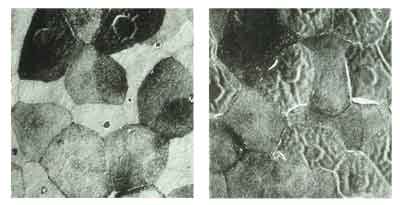
Figure 1, at left, a scanning electron micrograph of rabbit corneal epithelium after mild dosing with 0.02% benzalkonium chloride. In Figure 2 (right), after exaggerated dosing with 0.02% BAK, there is an increase in epithelial holes, a loss of peripheral microvilli and a noticeable wrinkling of surface cells.
Dry eye is a condition of abnormal or insufficient tears. This induces a state of reduced tear clearance and increased osmolarity, causing ocular irritation. Irritation leads to the release of proinflammatory cytokines that cause inflammation and eventual damage to ocular structures such as the corneal epithelium. Not only does this produce discomfort and visual degradation, it also decreases the ability of glycocalyx, present on epithelial cell membranes, to bind mucins onto the corneal surface, increasing tear film instability. Improper tear spreading or an increase in tear evaporation rate results in direct exposure of the ocular surface, compounding dry eye complications.
Dry eye may also result from the use of medications that cause abnormal or insufficient tear production, meibomian gland disease and/or mucin abnormalities, or a decrease in the tear film breakup time (TBUT) to less than the rate of blinking.
Common manifestations of dry eye include burning, itching, watery eyes, irritation, and redness. Many of these are exacerbated by a confluence of environmental and behavioral factors, such as climate, humidity and visual attentiveness. When patients experience these symptoms, often accompanied by inflammation, they may seek relief by using over-the-counter artificial tears. However, artificial tears contain many of the same potentially toxic preservatives that can, over time, aggravate the symptoms of OSD. Cumulatively, these factors may lead to epithelial cell damage that causes further discomfort and visual degradation.
Despite its high prevalence, OSD is an underappreciated problem in the glaucoma population. The lack of awareness may be due to practitioner distraction by other patient problems, lack of time in busy clinical practice, unfamiliarity with the problem and its consequences, or other factors. Nonetheless, it is important for the eye care provider, who often serves as the first option for patients seeking treatment for ocular discomfort, to screen vigilantly for OSD in patients who are at risk.

Common signs of OSD (See Table 1) include, but are not limited to, the following: abnormalities of the tear film and TBUT; meibomian gland dysfunction; turbid secretions; lid margin vascularization, laxity or irregularity; and corneal or conjunctival staining.9 Simply by flipping the lower eyelid, the clinician can quickly observe mild-to-moderate signs of OSD that may eventually lead to meibomian gland obstruction or disease. Slit-lamp examination can be used to examine the eyelids, margins, glands, and the conjunctival vasculature and epithelium, as well as TBUT.
A diagnosis of OSD can then be confirmed by using fluorescein stain, lissamine green or rose bengal staining. Fluorescein is a yellow dye that, under blue light, indicates areas of corneal damage. It can also be used to assess TBUT. Lissamine green is another stain that can be used to assess OSD. This stain adheres to dry areas on the cornea, often within five seconds, indicating a compromised epithelium. Lissamine green also stains cells that are in the early stages of conjunctival and epithelial damage. Rose bengal is a stain that is taken up by damaged epithelial cells for visualization by the practitioner, but may be more irritating to the eye than lissamine green. The Schirmer test is an alternative method of diagnosis, although this method has fallen out of favor recently due to logistic difficulties and poor sensitivity.
Because the ocular surface can be affected by these tests, it is important to screen patients rationally and systematically to identify those most at risk for OSD.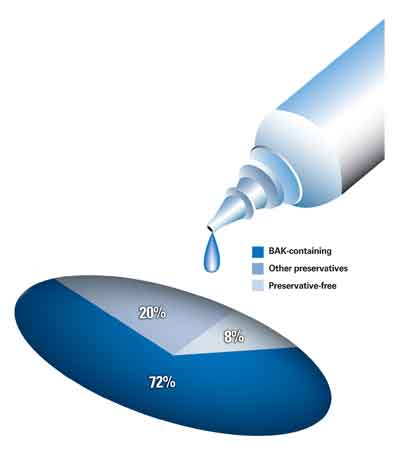
Chronic BAK Use in Glaucoma
Ophthalmic preservatives help prevent bacterial contamination and prolong shelf life by limiting biodegradation and maintaining drug potency. All multidose ophthalmic preparations are mandated by the U.S. Food and Drug Administration and the U.S. Pharmacopoeia to contain a preservative to maintain a nonhazardous level of contamination. When used chronically, however, preservatives can disrupt the precorneal tear film and lead to damage to the epithelial surface, cornea, and conjunctiva and worsening of OSD and its symptoms.13
Benzalkonium chloride is the most common preservative used in commercially available eye drops (See graphic above). A quaternary ammonium compound with cationic surfactant properties, BAK acts on microorganisms by altering the permeability of cell membranes and causing the lysing of cytoplasmic contents. It is both bacteriostatic and bacteriocidal, prevents bacterial, fungal, and amoebal growth, and inhibits bottle contamination and colonization with active pathogens of ocular infection. By preventing decomposition of the active ingredients at both room and elevated temperatures, BAK also acts as a preservative. It is non-selective in its effect on cell membranes and, therefore, may affect permeability of human cell membranes. It has also been shown to enhance corneal penetration of some drugs by causing a separation of the epithelial cells.
While BAK in an ophthalmic product's formulation engenders several positive attributes, it can also cause dose-dependent detrimental effects on healthy ocular tissue. At a BAK concentration of 0.0001 percent, arrest of cellular growth takes place.3 A BAK concentration of 0.01 percent induces cellular apoptosis, and at a concentration of 0.05 to 0.1 percent, necrosis occurs.13
Short-term exposure to BAK alters the precorneal mucin, which is important in maintaining the integrity of the tear film.14 BAK reduces TBUT.15,16 This effect contributes to dry eye, reduces the rate of removal of other noxious substances, and counteracts the protective effects of tears to the cornea. Particularly with chronic exposure, BAK also decreases the integrity of epithelial cells, compromising the epithelial barrier and impairing the rate of corneal and conjunctival healing.7,17
The impact of BAK on corneal epithelium is depicted in Figures 1 and 2. An increase in epithelial holes, a loss of peripheral microvilli, and a noticeable wrinkling of corneal surface cells are observed after BAK dosing. In addition, BAK lowers the density of goblet cells in the conjunctival epithelium. This action contributes to chronic, subclinical inflammation, immune cell and fibroblast deposits, and cytokine release. BAK also augments the accumulation of inflammatory markers in the trabecular meshwork, contributing to fibroblastic changes.7 Furthermore, evidence suggests a possible correlation between BAK and cataracts,18 as reported in three large, randomized controlled trials.19-21 Finally, in addition to cytotoxicity, preservatives can induce hypersensitivity, or allergic, reactions.10
Although there is increasing evidence that chronic exposure to BAK may have deleterious effects, it is still the most commonly used preservative in commercially available ophthalmic products. Glaucoma products contain different concentrations of BAK (See Table 2). The cumulative exposure to BAK can exacerbate OSD, particularly in glaucoma patients. In fact, long-term use of ophthalmic preparations for the treatment of glaucoma has been associated with toxic and inflammatory changes of the ocular surface.7,22 Furthermore, patients with dry eye may not produce sufficient tears to dilute the effects of a toxic preservative on the corneal surface.
It has been demonstrated that BAK affects corneal, conjunctival, and trabecular integrity, and may contribute to the development of cataracts.18-21 Furthermore, BAK may also predispose a patient to a greater risk of trabeculectomy failure,10,23 a surgical treatment option for the management of glaucoma. Finally, toxicity of topical ophthalmic medications may compromise the healing of wounds from other direct or indirect sources.24

Because of their potent efficacy, prostaglandins are a mainstay in the treatment of glaucoma. Of these analogues, latanoprost (Xalatan; Pfizer) contains 0.02% BAK, the highest concentration among the commercially available prostaglandins.25 Studies indicate there are BAK dose-dependent conjunctival and corneal epithelium toxicities that clinicians will need to take into consideration when using this agent in their glaucoma patients, especially in those who have OSD. J.M Guenoun and colleagues have shown that latanoprost produces a significant decrease in conjunctival epithelial cell membrane integrity and a significant increase in apoptosis when compared to another prostaglandin analogue containing 0.005% BAK.22 Richard Yee, MD, and colleagues have reported that latanoprost causes significantly more cell death of human corneal epithelial cells when compared to travoprost without BAK (Travatan Z, Alcon).26 Considering the role of latanoprost in the management of glaucoma, a BAK-free alternative would be desirable to minimize the issues associated with chronic exposure.
Several aspects of glaucoma therapy may exacerbate the problems associated with BAK exposure. For example, topical ophthalmic treatment for glaucoma is chronic; these medications must be used daily to realize the maximal benefit. Research demonstrates that longer BAK exposure increases corneal epithelial cell lysis.11 Furthermore, patients with glaucoma are often using multiple topical eye drops to treat the disease and its symptoms. These factors will likely increase their regular exposure to BAK. Other considerations that may influence corneal toxicity include: concentration of BAK in the ophthalmic drops selected for treatment; the number of drops used per dose and per day; the formulation of the preparation; the concentration of the active ingredient; patient age (older patients are more likely to suffer from dry eye and OSD); concomitant disease states that require treatment with other topical ophthalmic preparations; the pH of the eye drops selected for treatment; and contact lens use, as soft contact lenses have the ability to absorb many topical products and prolong their effects. Exposure to BAK, and its associated adverse effects, may also be exacerbated if the patient is using other preservative-containing ophthalmic products for other indications such as allergies, infection, or surgery. The collective effects of these adverse consequences may lead to decreased patient compliance16 and exacerbation of OSD.
All factors considered, though BAK has served as an effective preservative, the accumulating evidence indicates that it may be time to seek out alternatives for use in products for the treatment of chronic ocular diseases.
Alternatives and Potential Solutions
Therapy for OSD and dry eye includes nonpharmacological actions such as modification of environmental and behavioral aggravators and the use of artificial tears. Unfortunately, these measures are often insufficient or inconvenient for many patients, and other treatment options, such as a topical immunomodulator or punctal occlusion, become necessary in patients with moderate to severe disease. On the basis of the evidence presented above, one goal, then, is to reduce exposure to BAK. To accomplish this, topical medications must be formulated with either no preservative or a preservative other than BAK.
One currently available option to address the problems posed by BAK is preservative-free products. For example, preservative-free artificial tears, such as Systane PF (Alcon) or Refresh Plus (Allergan), are available in unit-dose containers. For the treatment of glaucoma, a preservative-free timolol formulation (Timoptic in Ocudose; Merck) is also available in unit-dose containers. The use of preservative-free products requires production, use, and disposal of single-use vials. For many patients, one multidose vial is a much more convenient alternative to several preservative-free unit-of-use vials, which may become cumbersome, especially for those medications requiring multiple administrations per day.
Another potential solution to the BAK problem is a product that uses a novel ionic buffer preservation system. Systane Free Lubricant Eye Drops (Alcon) utilizes an ionic buffer system to achieve its preserving action based on four ingredients: borate; aminomethyl propanol (AMP); sorbitol; and zinc. Systane Free provides the required preservation efficacy in the bottle while remaining gentle to the ocular surfaces. Upon contact with the eye, the preservation system becomes inactive, providing a preservative-free solution for dry-eye patients.27 (Hoffman H, et al. IOVS 2006;47:ARVO E-Abstract 5588) For glaucoma therapy, Alphagan-P (Allergan) is a brimonidine tartrate formulation that uses Purite as a preservative.
A prostaglandin alternative to Xalatan is Travatan Z (Alcon), a new formulation of travoprost. This product uses the what Alcon calls the "sofZia" preservative system and should be available for clinical use in the near future.28 A recent multicenter trial has shown that Travatan Z is equally efficacious and associated with a lower rate of patient-reported hyperemia compared to the commercially available Travatan solution, which is preserved with BAK.29
Finally, if tears or non-BAK formulated products cannot address the problem adequately, then another alternative is laser or incisional surgery for glaucoma treatment. However, many of these patients may end up on glaucoma medication. The decision regarding what is the appropriate strategy should carefully consider the patient's individual needs as related to control of glaucoma and ocular surface disease.
The problems associated with the use of BAK have driven clinicians to seek alternatives, particularly among glaucoma patients with OSD. Community eye-care providers, who are potentially the first-line in recognition of OSD and the problems associated with BAK, should be aware of the needs of these patients. The clinician should attempt to identify patients with particular risk factors and signs or symptoms of OSD. Such patients include the elderly and those chronically using many BAK-containing products.
Newly developed BAK-free medications offer an excellent alternative for the treatment of glaucoma, especially in those patients with OSD or with multiple eye-drops. Hopefully, these products will contribute to a new, safer, BAK-free era in the medical management of glaucoma.
Dr. Whitson is an associate professor of ophthalmology at the University of Texas Southwestern Medical Center, Dallas. Contact him at UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvd, Dallas, Texas, 75390-9057. Phone: (214) 648-4733, e-mail: jwhits@ yahoo.com.
Dr. Varner is a consultant for Akita Biomedical Consulting, San Clemente, Calif. Phone: 630-963-0129, e-mail: dwvarner77@yahoo.com.
Dr. Netland is a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, Tenn. Phone: 901-448-2629, e-mail: mesmith@utmem.edu, pnetland@dellmail.com.
1. Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol 2002;506(Pt B):989-98.
2. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003;136:318-26.
3. Tsai JH, Derby E, Holland EJ, Khatana AK. Incidence and prevalence of glaucoma in severe ocular surface disease. Cornea 2006;25:530-2.
4. Alcon Laboratories, Inc. Global Marketing Intelligence Survey. December 14, 2005.
5. Yu JY, Wu E, Kahook MY, Noecker RJ. Assessment of prevalence of dry eye among glaucoma patients. Poster accepted for presentation at American Academy of Ophthalmology. November 11-12, 2006; Las Vegas, NV.
6. Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Experiment Ophthalmol 2000;28:113-7.
7. Baudouin C, Pisella PJ, Fillacier K, Goldschild M, Becquet F, De Saint Jean M, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: Human and animal studies. Ophthalmology 1999;106:556-63.
8. Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 2005;31:186-93.
9. American Optometric Association. Care of the patient with ocular surface disorders. 2nd ed. St. Louis (MO): April 2003. Available from: http://www.aoa.org/documents/CPG-10.pdf.
10. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 2002;86:418-23.
11. Cha SH, Lee JS, Oum BS, Kim CD. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol 2004;32:180-4.
13. De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feltmann G, Baudouin C. Effects of BAK on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci 1999; 40:619-30.
14. Chung SH, Lee SK, Cristol SM, Lee ES, Lee DW, Seo KY, et al. Impact of short-term exposure of commercial eye drops preserved with benzalkonium chloride on precorneal mucin. Mol Vis 2006;12:415-21.
15. Baudouin C, de Lunardo C. Short term comparative study of topical 2% carteolol with and without benzalkonium chloride in healthy volunteers. Br J Ophthalmol 1998;82:39-42.
16. Ishibashi T, Yokoi N, Kinoshita S. Comparison of the short-term effects on the human corneal surface of topical timolol maleate with and without benzalkonium chloride. J Glaucoma 2003;12:486-90.
17. Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea 2004;23:490-6.
18. Brandt JD. Does benzalkonium chloride cause cataract? Arch Ophthalmol 2003;121: 892-3.
19. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268-79.
20. Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120:701-13.
21. Leske MC, Wu SY, Nemesure B, Hennis A; Barbados Eye Studies Group. Risk factors for incident nuclear opacities. Ophthalmology 2002;109:1303-8.
22. Guenoun JM, Baudouin C, Rat P, Pauly A, et al. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci 200546:2444-50.
23. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol 1994;112:1446-54.
24. Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication . I. The conjunctival cell profile. Arch Ophthalmol 1994;112:1437-45.
25. Pfizer, Inc. Xalatan (latanoprost ophthalmic solution) package insert. New York, NY: September, 2003.
26. Yee RW, Norcom EG, Zhao XC. Comparison of the relative toxicity of travoprost 0.004% without BAK and latanoprost 0.005% in an immortalized human cornea epithelial cell culture system. Adv Ther. In press.
27. Rodeheaver D, Griffin J, Hendrix C, et al. Pre-clinical evaluation of a novel artificial tear (AT) for dry eye. Presented at: 4th International Conference on the Lacrimal Gland, Tear Film, Ocular Surface and Dry Eye Syndromes: Basic Science and Clinical Relevance; November 17-20, 2004; Fajardo, Puerto Rico.
28. Alcon Laboratories, Inc. Travatan Z (travoprost ophthalmic solution) package insert. Fort Worth, Texas: August 2006.



