Here, to shed light on this topic, three surgeons with extensive experience developing and using these formulas share their thoughts.
Formula Evolution
As our understanding of the eye’s anatomy has improved, the complexity of the predictive formulas for IOL power has increased. That’s not the result of a change in our understanding of optics; it’s because of refinement in our ability to predict where the IOL will sit inside the eye. “All the theoretical formulas use the same vergence formula to relate the cornea, the lens and the distances between them,” says Jack T. Holladay, MD, MSEE, FACS, clinical professor of ophthalmology at Baylor College of Medicine in Houston and developer of the Holladay I and Holladay II formulas. “That vergence formula was first published by Stanilov Fyodorov back in 1975.1 The only thing that’s different [among the current theoretical formulas] is the prediction of the effective lens position. Prior to about 1970, everybody used about 4.5 mm for the axial length of every eye. Then Richard Binkhorst, MD, suggested that if the axial length is 10 percent longer than average, we should use a value 10 percent greater than 4.5 to calculate the ELP, and if the eye is 10 percent shorter, a value 10 percent smaller. He was the first person to individualize this.
 |
“For many years, most of the formulas, including the Holladay I, SRK/T and Hoffer Q, just required inputting axial length and K-reading, because those were the measurements we had back then,” he continues. “Then Thomas Olsen, MD, PhD, came out with a formula using four predictors: axial length; K-reading; anterior chamber depth; and lens thickness. In 1992 our team created the Holladay II formula, using seven variables to predict the effective lens position. Then Olsen and Barrett followed with new seven-variable formulas of their own. These formulas use axial length, K-reading, anterior chamber depth, lens thickness, corneal diameter, refraction and some, like the Holladay II, the age of the patient.”
“IOL power calculation formulas have come a long way,” agrees Uday Devgan, MD, FACS, FRCS, in private practice at Devgan Eye Surgery in Beverly Hills, chief of ophthalmology at the Olive View UCLA Medical Center and clinical professor of ophthalmology at the Jules Stein Eye Institute in Los Angeles. (Dr. Devgan helped to develop the Ladas Super Formula.) “For a long time people were using what are called the third-generation formulas—Hoffer Q, Holladay I and SRK/T. Those were the first to go beyond the most basic notions about how to calculate IOL power. Over time, surgeons reached a general consensus about how to use these formulas: The Holladay I formula produces the most accurate results with average eyes; the Hoffer Q works best with shorter eyes (hyperopic, small eyes with a short axial length); and the SRK/T formula produces the best results with longer eyes. So surgeons used whichever formula seemed most appropriate for the eye in question.
“Eventually, surgeons developed fourth-generation formulas which incorporate additional data,” he says. “The Haigis formula requires the K-reading and the axial length, but also requires the anterior chamber depth. The Holladay II formula incorporates seven variables. Then people began suggesting fudge factors to refine the outcomes even further. Doug Koch and Li Wang from Baylor College of Medicine published a very good study about axial length adjustment in highly myopic eyes. They provided a factor [the Wang-Koch modification] to apply to your measurements if the eye is more than 25 or 26 mm long.
“By this point,” he notes, “the formulas had become very convoluted. I literally had a flow chart on my desk: If this, then that. If that, then this. It became very cumbersome.”
Trying to Simplify
Dr. Devgan says the newest formulas are trying to find ways to simplify the calculation process without giving up any accuracy. “Graham Barrett has created the Barrett Universal Formula, which is meant to work for all eyes,” he says. “Hill-RBF refers to a radial basis function; it’s a big-data/neural-net-based formula, incorporating data from thousands of eyes. It’s not a specific equation; instead, it’s a method of using existing data to predict results for your set of measurements.
“Finally, the Ladas Super Formula incorporates many existing formulas into a single equation that shifts your measurements into the right formula automatically,” he explains. “If you’re dealing with a short eye, the Super Formula uses the equations that get the best results with short eyes to generate a result. It also incorporates data from 100,000 surgeries and constantly improves its accuracy based on new data that’s coming in. The addition of crowd-sourced data lets you be pretty darn accurate. It can also give you an answer based on data from your own past results. But any of the latest formulas, including the Ladas Super Formula, the Barrett Universal II and the Hill-RBF, should give surgeons great results, as long as they’re paying close attention to the details.”
Is One Formula the Best?
This is a tricky question to answer, in part because some formulas seem to give better results in certain types of eyes (short vs. long eyes, for example), and in part because not much research has been done comparing formulas.
The development of each formula has been different as well. “Everyone who develops a formula says his is the best,” notes Dr. Holladay. “It’s not necessarily that people are biased; it’s that formulas are developed based on a set of data, and the formula is refined until it produces the best outcomes—with that dataset. If you develop your formula using 300 cases, your formula is always going to be the best with that dataset because you’ve tweaked your formula until it produced the best results. Whether it will produce the best results with every other set of cases is a different question.”
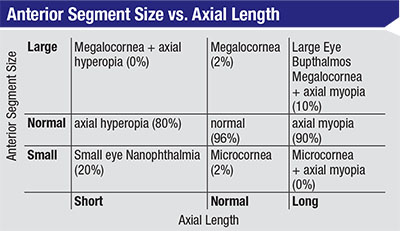 |
| Surgeons have traditionally assumed that short eyes have shallow anterior segments and long eyes have deep anterior segments, but research by Jack Holladay, MD, MSEE, FACS and James Gills, MD, found that this isn’t the case. |
“There aren’t many studies comparing recent formulas such as the Barrett Universal II and Hill-RBF,” notes Douglas D. Koch, MD, professor of ophthalmology at Baylor College of Medicine in Houston. (Dr. Koch and Li Wang, MD, PhD, created the Wang-Koch modification.) “The best data I’ve seen was more than 90 percent of eyes within 0.5 D of predicted outcome for the Hill-RBF in normal eyes. I also still find the Holladay I to be an excellent formula, and I now also routinely use the Barrett Universal II. I’ve seen data that suggests that the Barrett Universal II formula does very well in long eyes, but perhaps not quite as well as the Holladay I formula combined with our Wang-Koch modification. In short eyes, we have some preliminary data that suggests that the Hill-RBF formula is a little better than some other formulas. It’s also important to point out that both the Hill-RBF and Barrett are constantly being evaluated for ongoing improvement. Overall, they’re all pretty close.”
Dr. Devgan says he’s had great success using the Ladas Super Formula that he helped to develop. “We’re able to get 90-plus percent of eyes within half a diopter,” he says. “The older, third-generation formulas usually only get about 70 percent within a half diopter. That’s a big difference. In addition, I can now outsource the lens calculations to someone else in my practice. We just plug the numbers in and the calculation is done. Right now we have a Ladas Super Formula program that can be installed on your biometer that will automatically import the data to eliminate transcription errors. We’re also working on adding character recognition technology to the Ladas Super Formula app we’re developing, so you’ll be able to use your phone to take a photo of the printout from the IOLMaster or LenStar and it will automatically pick up all of the data.”
Dr. Devgan also appreciates being able to base the result on either crowd-sourced data or his own previously entered data. “I’ve entered data from a thousand eyes of my own,” he notes. “I know that my results with myopic eyes tend to be pretty spot on, so I can just use my own previous results to determine what I should do. When I’m operating on an unusual eye where I don’t have many previous data points to work with, I can use the data others have turned in when operating on similar eyes. I might see an eye like that once every five years, but with input from thousands of surgeons, the formula has plenty of data to work with.”
Dr. Devgan points out that surgeons wanting to try the Ladas Super Formula can access it for free at IOLcalc.com. “An app with the formula is coming soon for both the iPhone and Android devices,” he says. “The formula can also be installed on an instrument like the LenStar or IOLMaster.”
Why Use Seven Variables?
“The reason it makes sense to use a formula with seven variables is that the more you know about the anatomy of the eye and the patient, the better you’ll be able to predict the effective lens position,” explains Dr. Holladay. “Here’s a specific example involving the Holladay II formula. Our group was working with James Gills, MD, on a group of eyes that needed lenses between 40 and 60 D because the eyes were so short—from 15 to 20 mm long. The outcomes we achieved were mixed. Some we got right; others we missed by as much as 6 D.
“To try and figure out the reason for this, we went back and measured six anatomic characteristics of these eyes,” he says. “We found that the white-to-white measurement—the corneal diameter—was the most helpful of the six measurements in terms of explaining the discrepancy. We discovered that these very small eyes fell into two categories: Some short eyes had small anterior segments and small white-to-white measurements; but other short eyes had a normal anterior segment rather than a small one. The latter eyes also had corneal diameters of 11.8 or 12 mm.
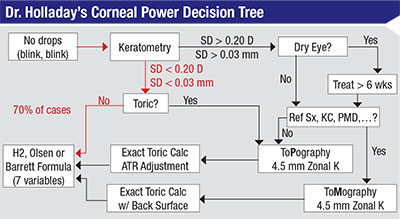 |
| Jack Holladay, MD, MSEE, FACS, recommends using this decision tree to determine the power of the lens you should implant to get as close as possible to your desired outcome. The majority of eyes should follow the decision path marked in red. |
Dr. Holladay has created a chart that illustrates this point [above]. “You can divide eyes along two axes: the axial length and the size of the anterior segment,” he says. “That leaves you with nine categories, ranging from long eyes with shallow anterior segments to short eyes with deep anterior segments. Most people have traditionally assumed that eyes fall into the boxes along the diagonal—that short eyes have shallow anterior segments and long eyes have deep anterior segments. But as our measurements found, that’s only true of short eyes 20 percent of the time, and only true in long eyes 10 percent of the time. Eighty to 90 percent of the time, both types of eyes have a normal anterior segment depth. That’s one of the mistakes surgeons make with the two-variable formulas; those formulas assume that short eyes have shallow anterior segments, which is only true 20 percent of the time. The seven-variable formulas correct for that mistake.
“Once we took this into account, all of a sudden we were no longer making those 6-D errors,” he says. “We got the eyes that were proportionally small right, and the eyes that had big anterior segments but short posterior segments right as well. So having the extra measurements can make a big difference in many eyes. In fact, studies conducted by the lens manufacturers, as well as independent groups, have confirmed that the seven-variable formulas produce the most accurate results.2-14 Of course, the lenses those formulas recommend may not be exactly the same because the datasets used to develop them were different. But when applied to new datasets, there’s no statistically significant difference that would allow you to say that, overall, one formula is better than another.”
If a seven-variable formula like the Holladay II is more accurate, does it still make sense to use the Wang-Koch modifier? “We’ve verified that the Holladay I version of the Wang-Koch formula works equally well for the Holladay II, so we use the Wang-Koch modification for all eyes 25.2 mm and longer,” says Dr. Koch. (Dr. Holladay, incidentally, agrees that the Wang-Koch modification increases accuracy when dealing with long eyes. He believes the need for compensation results from optical biometers producing exaggerated measurements when the eyes being measured exceed 25 mm.)
When Dr. Holladay teaches classes in this subject, he offers his students two basic pieces of advice. “Number one,” he says, “personalize your constant; otherwise there’s no reason to worry about the formula because your result will be off anyway. Second, the seven-predictor formulas are the best, although there’s no clear winner among them; each one produces a slightly different recommendation, and which is the best depends on your dataset. If you use a seven-predictor formula and personalize your constant, you’ll get more than 90 percent of your cases within half a diopter.”
Accurate Input Counts
Dr. Koch points out that almost all formulas are subject to two sources of uncertainty: the method the formula uses to calculate the ELP, and the accuracy of the measurements you’re putting into the formula. “This is especially true of corneal measurements,” he says. “I measure with two biometers and look at three sets of K-readings from different devices, but I still sometimes get postoperative refractive errors greater than half a diopter. In my Jackson Memorial lecture at the American Academy of Ophthalmology meeting this year, I gave an example of this. The K-reading I measured for one patient was 44.1, and the patient ended up myopic postoperatively. I remeasured the patient two weeks later and got a reading of 44.6, which was enough of a difference to account for the error. Even with careful measuring and our advanced technology, this can still happen. You just have to do your best and make sure your patients understand that the outcome won’t always be what you expect it to be.”
Dr. Koch says both the LenStar and IOLMaster produce good results when used correctly. “I use both,” he says. “I do prefer the IOLMaster 700 to the IOLMaster 500; I find the corneal power measurements are superior, and the 700 has greater accuracy when measuring anterior chamber depth. The IOLMaster 700 is also the most robust biometer for measuring axial length in eyes with dense cataracts. One limitation of the 700, however, is that while it includes the Holladay II formula, it doesn’t include the Barrett or Hill-RBF formulas. The LenStar offers all three, along with a version of the Olsen formula that’s not quite as sophisticated as the one you can purchase directly from Dr. Olsen. I presume the IOLMaster will expand their formula options as time goes by. In the meantime, if you have the IOLMaster and want to use all three formulas, you can use the Barrett and Hill-RBF formulas online at the ASCRS website.
“Whichever instrument you use, it’s important to look at the quality and consistency of the data,” he continues. “With the IOLMaster, you can look at the images that are reflected off the cornea from the instrument’s 18 LED lights; you may see that some of the reflections are smudgy instead of sharp. That tells you that the reading may not be as accurate as it should be. With the LenStar you can look at the standard deviations of its six corneal power measurements of the flat and steep meridians, as well as the astigmatic axis. With any measurement for IOL calculations, you need to validate the quality of the measurement.”
Dr. Koch says it’s also important to validate topography measurements, especially in terms of astigmatism. “It’s important to use a topographer that gives you information about the quality of the corneal surface,” he says. “I use the Gallilei topographer, which has both placido disc and Scheimpflug technology. It tells me a number of important things. First, it tells me if the mires are distorted, which means that the corneal surface is irregular and that I need to fix that before proceeding with surgery. Second, it gives me the magnitude and meridian of any astigmatism for comparing to the LenStar and IOLMaster measurements. Third, it tells me if there are any abnormalities in corneal curvature, any irregular astigmatism or other factors that might influence my IOL selection or my ability to safely use excimer laser surgery to treat the patient after I put in a premium IOL, should I need to do a postoperative adjustment.”
Holladay I or Holladay II?
Some surgeons report that they’ve gotten better results using the Holladay I formula than the Holladay II seven-variable formula. Dr. Holladay says that in his experience, this is because surgeons often input the refraction they measure at the preoperative visit. “That refraction has been altered by the cataract,” he explains. “The refraction helps the formula determine the size of the eye, but only if it’s the adult refraction before the cataract formed. Someone who was plano at age 21 might appear to be -4 D when measured right before cataract surgery. A person who really was -4 D as an adult before developing a cataract will have a thicker and more powerful crystalline lens than an average person. In those people, the lens you implant is going to end up in a different position than it will in an eye that was emmetropic before the cataract formed. If the eye you measure as -4 D right before the surgery was plano before the cataract, putting -4 D into the formula will throw your result off.
“Also, don’t use an average for something like lens thickness if you’re not able to measure it,” he continues. “If the eye isn’t average you’ll get a worse answer than if you just leave the number blank. As a rule, never put in values that you haven’t measured.”
Dr. Holladay admits that getting the patient’s previous refraction is more work. “That’s one reason many surgeons don’t do it,” he says. “If you’re seeing the patient for the first time, you have to ask the patient for help. He may still have the glasses he wore before he had LASIK. Or, you can ask questions like: ‘Were you able to get your driver’s license without wearing glasses?’ If the patient says yes, you know the patient was probably emmetropic. Many people can tell you how far away things had to be in order to see them clearly when they were in their 20s. Any of these things can give you a good approximation of the pre-cataract refraction. Just don’t use the manifest refraction measured on the visit before the cataract surgery.”
Formula vs. Intraop Refraction
Given that calculation formulas keep improving, some surgeons believe that intraoperative aphakic measurement—where the surgeon uses a tool like the ORA or HOLOS to take a refraction while the patient is on the operating table—may eventually become unnecessary. “I think better formulas and preoperative measurements will eventually reduce the indications for intraoperative aberrometry, but we’re not there yet,” says Dr. Koch. “That’s particularly true for astigmatism, and I still use this technology routinely for post-LASIK eyes. But I’m already using it a bit less than I used to.”
Dr. Holladay believes that no matter how good lens power formulas become, tools like intraoperative aberrometry will still be useful because of the accuracy limits of preoperative measurements. “The limit of our accuracy in lens calculation is the sum of the variability of all of the measurements we have to take,” he explains. “There will always be variability in the axial length measurement, and certainly in the K-reading, where we use a keratometer to measure the front radius of the cornea, assume that the back radius is 82 percent of that, assume an index of refraction and then calculate the power of the cornea.
“In contrast,” he continues, “intraoperative refraction simply uses the cornea as a lens to measure the aphakic refraction. That eliminates the variability of all of those calculations and assumptions, particularly for people who have had refractive surgery or have an irregular cornea.” (Dr. Holladay notes that in post-refractive surgery cases, the posterior surface radius is no longer 82 percent of the front surface radius, so measuring the back surface using tomography will increase the accuracy of your prediction.)
“We’ve spent 50 years doing intraocular lens calculations and we’re reaching a plateau at about 90 percent of eyes within half a diopter, in terms of accuracy,” he points out. “The limit isn’t the formula; the limit is the precision of the measurements that we put into the formula. If you look at it from an engineering perspective, the error of the answer is the sum of the squares of the error of each variable that goes into the formula. Our precision limit for the cornea is about 0.25 D; the error for axial length is about 0.1 mm; and the anterior chamber depth and the effective lens position is down to 0.2 or 0.3 mm. Those error limits keep us at about 90 percent of patients within half a diopter of the target outcome.”
Dr. Holladay acknowledges that the accuracy of these measurements may still improve. “That remains to be seen, however,” he says.
Dr. Devgan sees another possibility: incorporating an advanced formula into the intraoperative aberrometer. “Intraoperative aberrometry is more complicated than just the auto-refraction done on the table,” he explains. “All formulas calculate two things: the vergence calculation and the effective lens position. The aberrometers tell us the vergence calculation, but they still have to calculate the ELP, and that makes a huge difference in the outcome. That’s why the ORA requires you to input the K-reading, axial length and other biometric data before even starting the cataract surgery. So incorporating an advanced formula like the Ladas Super Formula into the intraoperative aberrometers should result in increased accuracy. It might be possible to get 99 percent of eyes within half a diopter.” REVIEW
Dr. Holladay is a consultant for AMO, NIDEK, Oculus, Acufocus, Allergan, Zeiss and Wavetec. Dr. Devgan is a principal in Advanced Euclidean Solutions which owns the Ladas Super Formula and the IOLcalc.com website. Dr. Koch consults for Alcon, AMO and Holos and has previously consulted for Carl Zeiss Meditec.
1. Fyodorov SN, Galin MA, Linksz A. Calculation of the optical power of intraocular lenses. Invest Ophthalmol 1975;14:8:625-8.
2. Trivedi RH, Wilson ME, Reardon W. Accuracy of the Holladay 2 intraocular lens formula for pediatric eyes in the absence of preoperative refraction. J Cataract Refract Surg 2011;37:7:1239-43.
3. Ghanem AA, El-Sayed HM. Accuracy of intraocular lens power calculation in high myopia. Oman J Ophthalmol 2010;3:3:126-30.
4. Bang S, Edell E, Yu Q, Pratzer K, Stark W. Accuracy of intraocular lens calculations using the IOLMaster in eyes with long axial length and a comparison of various formulas. Ophthalmology 2011;118:3:503-6.
5. Terzi E, Wang L, Kohnen T. Accuracy of modern intraocular lens power calculation formulas in refractive lens exchange for high myopia and high hyperopia. J Cataract Refract Surg 2009l;35:7:1181-9.
6. Lüchtenberg M, Kuhli-Hattenbach C, Fronius M, Zubcov AA, Kohnen T. Predictability of intraocular lens calculation using the Holladay II formula after in-the-bag or optic captured posterior chamber intraocular lens implantation in paediatric cataracts. Ophthalmologica 2008;222:5:302-7.
7. Hu BJ, Zhao SZ, Tseng P. Intraocular lens power calculation in cataract phacoemulsification after refractive surgery. Zhonghua Yan Ke Za Zhi 2006;42:10:888-91. Chinese.
8. Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg 2006;32:12:2050-3.
9. Chan CC, Hodge C, Lawless M. Calculation of intraocular lens power after corneal refractive surgery. Clin Experiment Ophthalmol 2006;34:7:640-4.
10. Wang L, Booth MA, Koch DD. Comparison of intraocular lens power calculation methods in eyes that have undergone laser-assisted in-situ keratomileusis. Trans Am Ophthalmol Soc 2004;102:189-96; discussion 196-7.
11. Wang L, Booth MA, Koch DD. Comparison of intraocular lens power calculation methods in eyes that have undergone LASIK. Ophthalmology 2004;111:10:1825-31.
12. Packer M, Brown LK, Hoffman RS, Fine IH. Intraocular lens power calculation after incisional and thermal keratorefractive surgery. J Cataract Refract Surg 2004;30:7:1430-4.
13. Gimbel HV, Sun R. Accuracy and predictability of intraocular lens power calculation after laser in situ keratomileusis. J Cataract Refract Surg 2001;27:4:571-6.
14. Seitz B, Langenbucher A. Intraocular lens calculations status after corneal refractive surgery. Curr Opin Ophthalmol 2000;11:1:35-46.



