At this year's meeting of the Association for Research in Vision and Ophthalmology, the members of the International Workshop on Meibomian Gland Dysfunction gave attendees a preliminary glimpse of their comprehensive report on the meibomian gland and its disorders. The report, which runs the gamut from the anatomy and physiology of the gland and proper definitions and classifications of meibomian gland dysfunction to thoughts on therapy, is the result of just over a year of research by the Tear Film and Ocular Surface Society.
"The objective of the workshop was to gather an international group, much like the TFOS Dry Eye Workshop, and discuss, using an evidence-based approach, the meibomian gland in health and disease," says Kelly Nichols, OD, PhD, chair of the Meibomian Gland Dysfunction Workshop. "Essentially, it's a very large survey report like you'd find in Survey of Ophthalmology or The Ocular Surface, but much larger."
The workshop is composed of seven subcommittees, each of which tackled a different aspect of the meibomian gland and its disorders. There is also an eighth subcommittee made up of representatives from the ophthalmic companies that sponsored the workshop.
Though the members of the workshop are keeping all of their findings under wraps until they can be formally published, most likely in Investigative Ophthalmology and Visual Science, they discussed some of the conclusions at ARVO.
Some of these findings involved a clear definition and classification of meibomian gland dysfunction by J. Daniel Nelson, MD, professor of ophthalmology at the
In his presentation, Dr. Nelson defined meibomian gland dysfunction as a "chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. This may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease." In discussing his presentation, Dr. Nelson notes that the TFOS workshop prefers the term hypersecretory MGD over the term "seborrheic dermatitis," which should help some clinicians differentiate the two conditions. "Seborrheic dermatitis is a skin condition, some suspect caused by a fungus," he says. "The confusion arises with the term seborrhea and hypersecretion.
While hypersecretion refers to increased/excessive meibum production, seborrheic dermatitis does not necessarily involve increased or excessive sebum production."
Dr. Nelson also addressed inflammation's role in MGD. "I don't think inflammation necessarily enters into the general classification, as it may be involved within any of the different conditions," he says. "For example, inflammation may or may not be seen in hyper- or hyposecretory states. However, it is important to recognize inflammation, since it influences what the therapeutic approach will be."
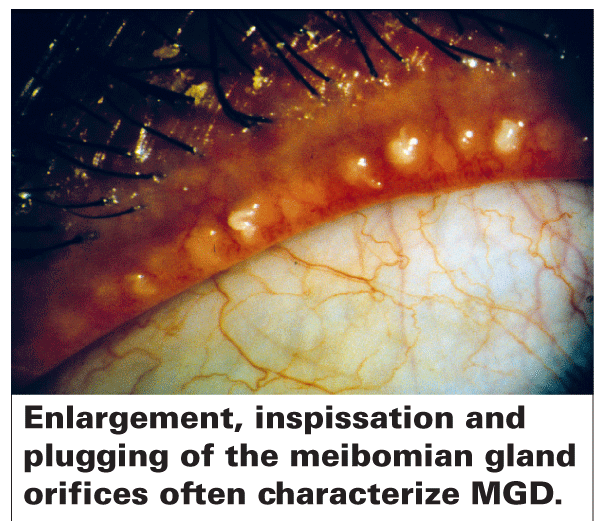
Many of the workshop's subcommittee reports have been finalized, but several are still being revised. Dr. Nichols says that when all the individual reports are ready they'll be submitted to IOVS, and will probably be published together in the September issue of the journal.
"Certainly, it's very timely," says Dr. Nichols. "There's a significant amount of interest given the number of pharmaceutical products being launched around the world and the continued interest in ocular surface disease in general. [The MGD Workshop] is the most expansive discussion of the meibomian gland to date."
BJO Reports Results of Macugen AMD Trial
The British Journal of Ophthalmology published online results from the LEVEL study evaluating Macugen (pegaptanib sodium) as a maintenance therapy in neovascular age-related macular degeneration. This large, open-label, uncontrolled, exploratory study enrolled 568 patients who had been treated one to three times for neovascular AMD, primarily with a non-selective VEGF-inhibitor such as ranibizumab or bevacizumab. During this induction phase, the mean visual acuity improved by 15.9 letters (49.6 letters to 65.5 letters). After entering the study, patients were switched to Macugen, a selective VEGF-inhibitor, with the possibility of using additional treatments if needed. At the end of this 54-week maintenance phase, mean final visual acuity was 61.8 letters. From the beginning of the induction phase to the end of maintenance phase, an approximately 16-month follow-up, 41 percent of patients gained at least three lines of visual acuity.
The study authors concluded that this induction-maintenance treatment strategy, using non-selective VEGF inhibitors then switching to Macugen, a selective VEGF inhibitor, may be an option for the long-term treatment of neovascular AMD. "A treatment protocol that combines the efficacy of a non-selective VEGF inhibitor with the safety profile of a selective VEGF inhibitor may be an attractive option, since elderly patients with AMD are already at increased risk of hypertension, stroke and other cardiovascular disease," said Thomas R. Friberg, MD, lead investigator of the LEVEL study and professor of ophthalmology and bio-engineering at the University of Pittsburgh.
"This treatment approach may be of particular interest to retina specialists and their AMD patients with cardiovascular co-morbidities who require long-term treatment with anti-VEGF drugs to manage their neovascular AMD," said Dr. Friberg. "Unlike non-selective VEGF inhibitors that should be administered monthly, Macugen is administered every six weeks. This substantially reduces the treatment burden on patients and their families."
Despite the limitations of an uncontrolled study, Macugen (Eyetech) when used as a maintenance therapy showed adverse events rates similar to those observed in the pivotal Phase III VISION trial at one year. During one year of follow-up, there was no evidence of increased risk of endophthalmitis, retinal detachment or traumatic cataract. The most common ocular adverse events were punctatekeratitis, eye pain and vitreous floaters. Reports of serious non-ocular, vascular events (including cardiovascular events) were rare.
LEVEL (EvaLuation of Efficacy and safety in maintaining Visual acuity with sEquential treatment of neovascuLar AMD) is a Phase IV, prospective, open-label, uncontrolled exploratory study designed to assess the efficacy of Macugen as a maintenance therapy in neovascular AMD patients who had at least one but not more than three prior treatments (induction phase) 30 to 120 days prior to study entry. Patients who showed significant clinical and/or anatomical improvement in their neovascular AMD, as determined by the study investigator, were enrolled in the LEVEL study and administered intravitreal injections of Macugen 0.3 mg every six weeks for 48 weeks with follow-up through week 54.
Additional treatments with other agents were allowed in the study eye at investigators' discretion for deteriorating AMD. Half of the patients (283/568) required an additional treatment during the study, which was given approximately five months post-baseline on average. Of those who received an additional treatment, 46 percent required only one such treatment.
The results from the exploratory trial suggest that this induction/maintenance regimen may be a promising approach in the long-term treatment of neovascular AMD. From the beginning of induction treatment to week 54 of the maintenance phase, 41 percent of patients gained >three lines of vision, 79 percent gained >0 lines and 92 percent lost <three lines of vision. Randomized, controlled trials are needed to corroborate the findings of the LEVEL study.

Study: Disclosure of Conflict Alone May Not Be Effective
Simple disclosure of funding sources ofclinical trials, as is commonly done in peer-reviewed medical literature, may not be effective in altering how physician readers interpret the results, says a study in the Journal of Medical Ethics.
Researchers randomly selected 515 fellows in the
The subjects did not significantly discount for conflicts of interest in their self-reported likelihood of prescribing the new drug after reading the single abstract and scenario.
Asked explicitly to compare conflict and no conflict, 69 percent reported that they would discount for researcher conflict and 57 percent said they would discount for presenter conflict. When asked to guess how favorable the results of this study were towards the new drug, compared with the other trials published so far, their perceptions were not significantly influenced by conflict of interest information.
While physicians believe that they should discount the value of information from conflicted sources, the authors conclude, they did not do so in the absence of a direct comparison between two studies.
J Med Ethics 2010;36:265-270



