Indocyanine green has been used in various medical applications, originally being developed as a possible blood substitute. For a number of years, ICG has been applied to ophthalmology as a dye for angiography to facilitate visualization of the choroid. More recently, ICG has been used in cataract surgery for staining of the anterior lens capsule and in vitreoretinal surgery to enhance visualization of tissues on the macular surface.
Early Uses, Advances
The primary intraoperative use for indocyanine green in vitreoretinal surgery is for staining of the internal limiting membrane in surgery for macular hole and macular pucker. In 1991, Neal Kelly and Robert Wendel originally reported successful macular hole repair with vitrectomy, separation of the hyaloid from the posterior retinal surface, and fluid-air exchange.1 Since that time, surgical technique has been modified in an effort to improve surgical results, including the use of adjuncts to facilitate hole closure2-5 and ILM peeling to reduce perifoveal tangential traction.6-10
ICG stains the ILM and facilitates visualization of the normally transparent tissue, making it easier to remove the ILM. Improved intraoperative visualization likely reduces surgical time and reduces the risk of mechanical injury to the retina. Although ICG does improve visualization, concerns exist regarding the safety of its use. Therefore, modifications of the use of ICG have been made to attempt to reduce the potentially toxic effects of ICG.
Surgical Techniques
ICG is used in vitreoretinal surgery following a complete vitrectomy and elevation of the posterior hyaloid when it is still adherent to the macula. There are various techniques for the use of ICG in macular hole surgery. In 2000, one group reported the use of ICG diluted in a viscoelastic substance to stain the ILM.9 Other techniques have included injection of the ICG solution into the fluid-filled vitreous cavity for varying lengths of time prior to ICG removal to allow for ILM staining. Also, some perform a near total fluid air exchange with installation of a small amount of ICG solution in the residual fluid overlying the posterior pole.
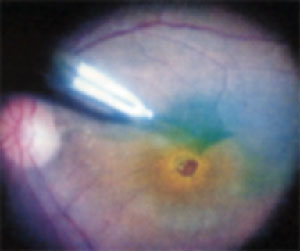
Once adequate staining of the ILM is obtained, an initial incision in the ILM is made using a microvitreoretinal blade. Because the staining is specific for the ILM, there is clear visualization of the interruption of the ILM with the initial incision as compared to the underlying retina. Next, a pick is used to elevate the edge of ILM to begin the dissection. Intraocular forceps are then used to remove the ILM from the retinal surface in a circumferential fashion around the macular hole. The area of ILM that is removed and the remaining ILM are clearly defined by staining with the ICG, thus facilitating the ability to achieve complete removal (See Figure 1).
|
|
| Figure 1. The indocyanine green-stained internal limiting membrane is easily identified and can be peeled from the retina surface with a microforceps. The ICG also clearly delineates the area around the macular hole where the ILM has been removed from the more peripheral area, where the ILM has not been peeled. |
Without the use of ICG, the ILM, which is relatively transparent, is difficult to identify and complete removal is often uncertain. Also, in cases with an unclear media, such as with a cataract or a cloudy cornea, ILM peeling may not be possible without the improved visualization afforded by ICG. Because ICG simplifies ILM dissection, efforts to find safe techniques for its use have been pursued.
Modifications in technique have primarily focused on efforts to reduce the potential toxicity of ICG. These modifications have included variations in the ICG concentration, limiting the staining time, and reduction of light intensity and exposure time. Protecting the hole with a viscoelastic substance to keep the bare retinal pigment epithelium from being exposed to the ICG solution also has been reported.11 An additional study has suggested that the elimination of sodium from the ICG solution may reduce the risk of RPE cell damage.12
Results
ICG has been shown to selectively stain the internal limiting membrane.13 The use of ICG to assist in ILM peeling has been shown to increase the macular hole closure rate as compared to macular hole repair without ILM peeling or with ILM peeling without the use of ICG.14
Although the macular hole closure rate is higher with the use of ICG, several reports have registered concern that the visual outcomes with ICG use have not been as favorable as compared to cases without the use of ICG. In one study, following macular hole surgery with and without ILM peeling and ILM peeling with ICG,14 the macular hole closure rate for non-ILM peeling cases was 85.4 percent. In cases with ILM peeling, the hole was closed in 85.7 percent without ICG and 100 percent in cases with ICG. Despite an improved closure rate using ICG, the visual outcomes were not as favorable for the ICG group. Both groups without ICG showed a statistically significant improvement in visual acuity, whereas the ICG group did not show significant visual improvement.
Other investigators reported a group of patients with 100-percent macular hole closure rate following ILM peeling with and without ICG, but the improvement in visual acuity was significantly better in the non-ICG group.15 An additional study suggested that the use of ICG may alter the cleavage plane for ILM dissection to involve more inner retinal layer cells which may be responsible for differences in visual results.16
ICG has also been used to facilitate membrane peeling for vitrectomy surgery performed for macular pucker. However, a report comparing the visual results following ICG-assisted ILM peeling versus non ICG ILM peeling in macular pucker showed less favorable visual outcomes following the use of ICG.17 The researchers reported visual improvement in 86 percent of patients without and 55 percent of patients with ICG-assisted surgery. These findings again raise the question of potential retinal toxicity rather than only RPE toxicity, as there is no macular hole present in these cases.
Concerns/Toxicity
Concerns exist regarding the safety of the use of ICG. Clinical reports have shown atypical retinal pigment epithelial changes following the use of ICG 18-19 (See Figure 2). Others have shown less favorable visual outcomes following the use of ICG.15-17 The exact mechanism for the potential toxicity is not known. Possible mechanisms include a direct toxic effect of ICG to the RPE cells and/or retina. In vitro studies have shown that exposure of ICG to cultured human RPE cells results in dose- and time-dependent damage to cellular structure.20 Also, it has been shown that ICG exposure results in alterations in cellular function in human RPE cell cultures.21 Another potential mechanism is enhanced phototoxicity. Although the use of ICG improves visualization of the ILM and should reduce surgical time and time of light exposure, ICG may reduce the "safe time" for light exposure, and therefore increase the potential for light toxicity. ICG has also been shown to be retained on the retinal surface and optic nerve for months after surgery and may pose long term risks for retinal toxicity.22
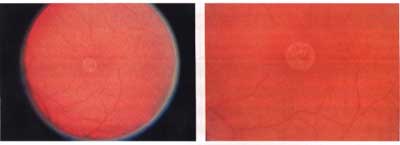 |
| Figure 2. Following macular hole surgery with ICG-assisted ILM peeling, there is a discrete circular zone of retinal pigment epithelium atrophy in the area of the previous macular hole and localized neurosensory retinal detachment. (Reprinted with permission from AJO 2002;133:89-94. Elsevier Science Inc.) |
The recent emphasis on ILM peeling for macular hole surgery has made the use of ICG commonplace in vitreoretinal surgery. ICG does facilitate the visualization of the ILM and therefore does make it easier to achieve more complete and consistent removal of the ILM. Although macular hole closure rates are higher with the use of ICG, visual outcomes may not be as favorable as ILM peeling without the use of ICG. The causes for the lack of visual improvement despite successful macular hole closure are not well understood.
While numerous surgeons have used ICG routinely for ILM peeling and have not observed adverse effects on anatomic and functional outcomes, concern does exist regarding the overall safety of its use. Clinical reports of atypical RPE changes following the use of ICG are disconcerting. Studies showing altered RPE cell structure and function following exposure to ICG in vitro bring the safety of the use of ICG into question. Modifications in technique including variations in the ICG concentration, amount of time for staining, and the use of viscoelastic substances to protect the bare RPE within the macular hole may reduce potential risks of ICG toxicity.
Alternative methods of improving visualization of the ILM have recently been reported. Early studies with the use of trypan blue have shown adequate staining of the ILM for visualization and no detrimental effects to the RPE or visual outcomes.23 Recently approved, trypan blue should soon be available for use in the United States. Additional reports have shown the triamcinolone acetate may be used to coat the macular surface and aid in visualization of the ILM during dissection.24
With the above concerns regarding the safety of the use of ICG, additional studies are needed to clarify the ultimate safety of its use. Potential alternatives such as trypan blue or triamcinolone should also continue to be pursued.
Dr. Engelbrecht is at the Barnes Retina Institute, Washington University School of Medicine, St. Louis, Mo.; Dr. Sternberg is at Vanderbilt Eye Institute. Contact Dr. Sternberg at 8030 Medical Center East, Vanderbilt University Medical Center, Nashville, Tenn. 37232-8808. Phone (615) 936-1453, fax: (615)-936-3497, or e-mail paul.sternberg@vanderbilt.edu.
1. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 1991;109:654-659.
2. Glaser BM, Michels RG, Kupperman BD, et al. Transforming growth factor-beta 2 for the treatment of full thickness macular holes. A prospective randomized study. Ophthalmology 1992;99:1162-1172.
3. Ligget PE, Skolik SA, Horio B, et al. Human autologous serum for the treatment of full thickness macular holes. A preliminary study. Ophthalmology 1995;102:1071-1076.
4. Korobelnik JF, Hannouche D, Belayachi N, et al. Autologous platelet concentrate as an adjunct in macular hole healing. A pilot study. Ophthalmology 1996;122:590-594.
5. Olsen TW, Sternberg Jr P, Capone Jr A, et al. Macular hole surgery using thrombin- activated fibrinogen and selective removal of the internal limiting membrane. Retina 1998;18:322-329.
6. Park DW, Sipperly JO, Sneed SR, et al. Macular hole surgery with internal limiting membrane peeling and intravitreous air. Ophthalmology 1999;106:1392-1398.
7. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol 2000;129:769-777.
8. Brooks Jr HL. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000;107:1939-1049.
9. Kadonosono K, Itoh N Uchio E, Nakamura S, Ohno S. Staining of the internal limiting membrane in macular hole surgery. Arch Ophthalmol 2000;118:1116-1118.
10. Burk SE, Da Mata AP, Snyder ME, et al. Indocyanine green assisted peeling of the retinal internal limiting membrane. Ophthalmology 2000;107:2010-2014.
11. Kusaka S, Oshita T, Ohji M, Tano Y. Reduction of the toxic effect of indocyanine green on retinal pigment epithelium during macular hole surgery. Retina 2003;23:733-734.
12. Ho JD, Chen HC, Chen SN, Tsai RJF. Reduction of indocyanine green-associated photosensitizing toxicity in retinal pigment epithelium by sodium elimination. Arch Ophthalmol 2004;122:871-878.
13. Gandorfer A, Messmer EM, Ulbig MW, Kumpik A. Indocyanine green selectively stains the internal limiting membrane. Am J Ophthalmol 2001;131:387-388.
14. Ando F, Sasano K, Ohba N, Hirose H, Yasui O. Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. Am J Ophthalmol 2004;137:609-614.
15. Horio N, Horiguchi M. Effect on visual outcome after macular hole surgery when staining the internal limiting membrane with indocyanine green dye. Arch Ophthalmol 2004;122:992-996.
16. Haritglou C, Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: A clinicophathologic correlation. Am J Ophthalmol 2002;134:836-841.
17. Haritoglou C, Gandorfer A, Gass CA et al. The effect of indocyanine green on functional outcome of macular pucker surgery. Am J Ophthalmol 2003;135:328-337.
18. Engelbrecht NE, Freeman J, Sternberg Jr P, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol 2002;133:89-94.
19. Maia M, Haller J, Pieramici DJ, et al. Retinal pigment epithelial abnormalities after internal limiting membrane peeling guided by indocyanine green staining. Retina 2004;24:157-160.
20. Ho JD, Tsai RJF, Chen SN, Chen HC. Cytotoxicity of indocyanine green on retinal pigment epithelium. Implications for macular hole surgery. Arch Ophthalmol 2003;121:1423-1429.
21. Sippy BD, Engelbrecht NE, Hubbard GB et al. Indocyanine green effect on cultured human retinal pigment epithelial cells: Implication for macular hole surgery. Am J Ophthalmol 2001;132:433-435.
22. Tadayoni R, Paques M, Girmens JF, et al. Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology 2003;110:604-608.
23. Vote BJ, Russell MK, Joondeph BC. Trypan blue-assisted vitrectomy. Retina 2004;24:736-738.
24. Kimura H, Kuroda S, Nagata M. Triamcinolone acetonide-assisted peeling of the internal limiting membrane. Am J Ophthalmol 2004;137:172-173.