Workup, Diagnosis and Treatment
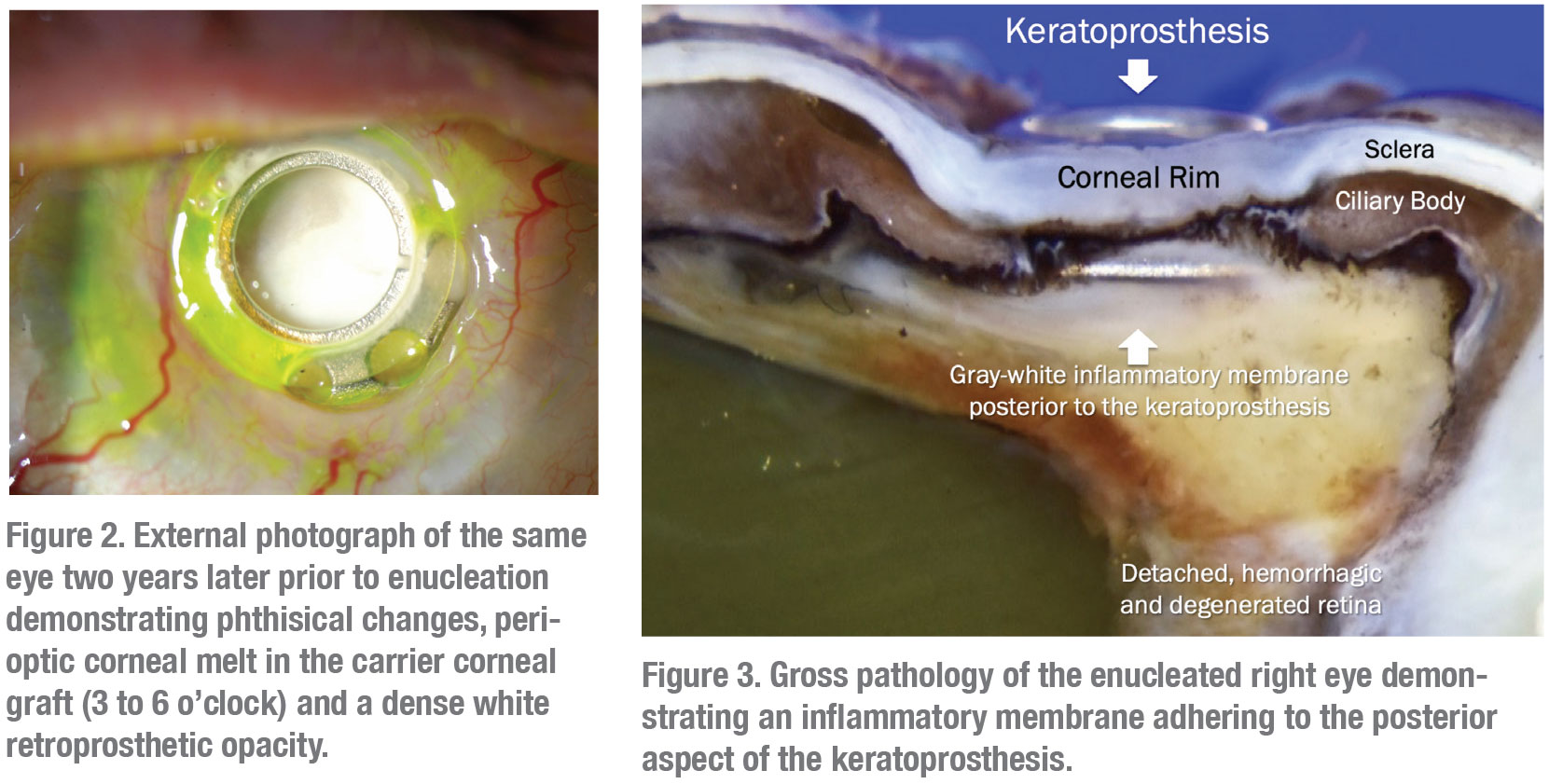 |
 |
The differential for the new retroprosthetic opacities included recurrent RPM, but suspicion was strongest for a smoldering infectious process, including both bacterial and fungal etiologies, especially in view of the fluffy appearance of the retroprosthetic opacities and vitreous involvement. The patient was referred to her retina specialist who proceeded with a pars plana vitrectomy, vitreous biopsy and injection of intraocular antibiotics and antifungals (vancomycin, ceftazidime and amphotericin). A diagnosis of chronic fungal endophthalmitis was established when cultures grew Candida parapsilosis.
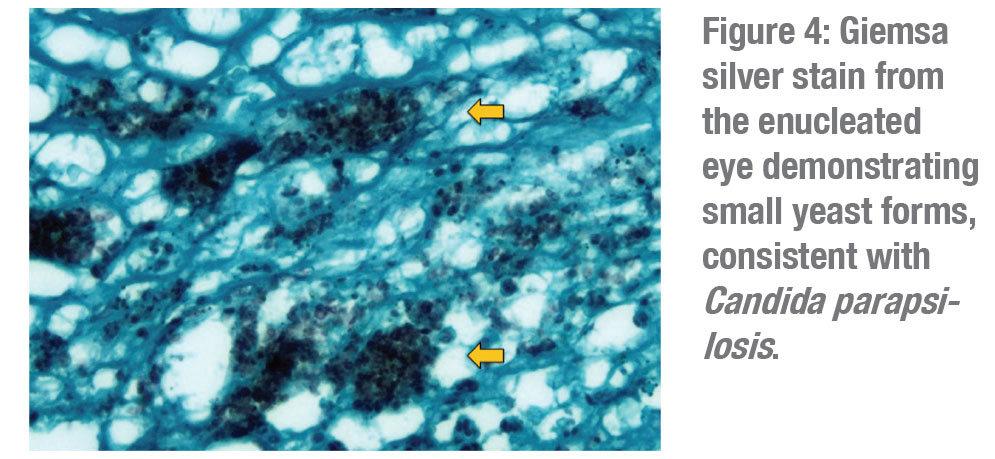
The patient was initially started on oral voriconazole by her retina physician and then switched to oral fluconazole. Despite these measures, the right eye developed peripheral proliferative vitreoretinopathy and a shallow peripheral retinal detachment on ultrasonography. Given the already limited visual potential, the patient opted for observation at that time. The retroprosthetic opacity continued to thicken and coalesce (Figure 2) and the eye eventually developed a funnel retinal detachment and progressed to phthisis over the next two years. The decision was made to enucleate the right eye. Pathology revealed a large grey-white inflammatory membrane behind the KPro (Figure 3) and Giemsa silver stain demonstrated small yeast forms compatible with Candida parapsilosis (Figure 4).
Discussion
The Boston KPro is the most frequently used keratoprosthesis, or synthetic cornea, with over 15,000 units implanted.1 This prosthesis consists of a back plate and optical stem that are incorporated with a donor cornea at the time of surgery. KPro implantation is primarily used when a standard PK is deemed unlikely to be successful, often after repeat PK failure.2,3
A 2015 review of the published literature on the safety and outcomes of Boston KPros found that development of a retroprosthetic membrane was the most common postoperative complication (30 percent), followed by glaucoma (27.5 percent).4 Other anterior postoperative complications included corneal melt and infectious keratitis. Infectious endophthalmitis was the most common posterior segment complication (4.6 percent) across all studies, although rates appeared to be down trending.4
KPro-related endophthalmitis occurs as virulent organisms from the ocular surface gain access into the eye through an altered barrier layer along the course of the implant. In bacterial KPro-related endophthalmitis, these causative organisms are often gram-positive cocci including various staphylococcus and streptococcus species.5 Prior to 1999, bacterial endophthalmitis was a formidable postoperative complication despite prophylactic antibiotic drops.5 In 1999, the addition of prophylactic topical vancomycin drastically reduced the rate of endophthalmitis due to its excellent gram-positive coverage. One 2009 Massachusetts Eye and Ear study of 255 KPro eyes demonstrated that the addition of prophylactic vancomycin resulted in a 0.35-percent risk of endophthalmitis per patient-year, 12 times less than eyes that didn’t receive vancomycin.6 A 2009 Wills Eye study of 37 KPro eyes found four cases of endophthalmitis, three of which admitted to discontinuing vancomycin prior to infection, further demonstrating the importance of prophylactic adherence.7
A 2015 literature review of fungal infections—and both keratitis and endophthalmitis—after KPro implantation found that fungal infection rates ranged from 0.009 to 0.02 infections per patient-year, with the largest single-surgeon study reporting a 10 year fungal endophthalmitis incidence of 2.4 percent.8 Candida species account for the majority of fungal infections in northern North America, with C. parapsilosis representing the most common isolate.9 C. parapsilosis is typically less virulent than other yeasts like C. albicans and C. tropicalis, however C. parapsilosis has a particular propensity to adhere to prosthetic materials, which explains its increased pathogenicity in KPro patients.10 Hypothetical risk factors for fungal infectious in KPro patients include prolonged soft contact lens wear and vancomycin prophylaxis,11 although some series on fungal keratitis refute these findings.12, 13
When fungal endophthalmitis is suspected in a KPro patient, a vitreous sample is obtained via vitreous tap or pars plana vitrectomy, together with injection of broad-spectrum intravitreal antibiotics and an intravitreal antifungal—followed by initiation of systemic antifungals.14-16 Although antifungal prophylaxis isn’t routinely practiced for the management of KPro patients, Massachusetts Eye and Ear does recommend considering burst antifungal prophylaxis (amphotericin B drops, twice per day for a week every three months) in high-risk patients and geographic locations.17
In conclusion, endophthalmitis is a serious complication following KPro implantation. Fungal endophthalmitis should be considered in patients with persistent or recurrent retroprosthetic opacities or those associated with vitreous involvement. C. parapsilosis is a common infectious agent in KPro-related fungal endophthalmitis due to its propensity to adhere to the prosthetic surface. REVIEW
1. Colby K, Dana R. Foundations of Corneal Disease: Past, Present and Future. 9th ed. Cham, Switzerland: Springer, 2019:115.
2. Ma JJ, Graney JM, Dohlman CH. Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin 2005;45:4:49-59.
3. Hannush SB, Riveroll-Hannush L. Postoperative Management of Boston Keratoprosthesis Type 1. In Mannis MJ, Holland EJ, eds. Cornea: Fundamentals, Diagnosis and Management. 4th ed. Elsevier, 2016:1644-1654.
4. Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI. Boston keratoprosthesis: Outcomes and complications: A report by the American Academy of Ophthalmology. Ophthalmology 2015;122:7:1504-11.
5. Nouri M, Terada H, Alfonso EC, Foster CS, Durand ML, Dohlman CH. Endophthalmitis after keratoprosthesis: Incidence, bacterial causes and risk factors. Arch Ophthalmol 2001;119:4:484-9.
6. Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea 2009;28:8:896-901.
7. Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea 2009;28:9:989-996
8. Odorcic S, Haas W, Gilmore MS, Dohlman CH. Fungal infections after boston type 1 keratoprosthesis implantation: Literature review and in vitro antifungal activity of hypochlorous acid. Cornea 2015;34:12:1599-605.
9. Wagoner MD, Welder JD, Goins KM, Greiner MA. Microbial keratitis and endophthalmitis after the boston type 1 keratoprosthesis. Cornea 2016;35:4:486-93.
10. Weems JJ. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin Infect Dis 1992;14:3:756-66.
11. Barnes SD, Dohlman CH, Durand ML. Fungal colonization and infection in Boston keratoprosthesis. Cornea 2007;26:1:9-15.
12. Chan CC, Holland EJ. Infectious keratitis after Boston type 1 keratoprosthesis implantation. Cornea 2012;31:10:1128-1134.
13. Kim M, Yu F, Aldave A. Microbial keratitis after Boston type I Keratoprosthesis implantation: Incidence, organisms, risk factors and outcomes. Ophthalmology 2013;120:11:2209-2216.
14. Odorcic S, Sabeti S, Haas W, Durand ML, Dohlman CH. Fungal infections in boston keratoprosthesis patients: Lessons learned and novel developments on the horizon. Semin Ophthalmol 2016;31(1-2):71-84.
15. Robert MC, Moussally K, Harissi-dagher M. Review of endophthalmitis following Boston keratoprosthesis type 1. Br J Ophthalmol 2012;96:6:776-80.
16. Malhotra C, Jain AK, Aggarwal N. Fungal keratitis and endophthalmitis after implantation of type 1 keratoprosthesis. Oman J Ophthalmol 2018;11:1:62-64.
17. Walcott-Harris R, Chodosh J, Dohlman CH. Antimicrobial prophylaxis for life: As important as ever. Boston KPro News [Serial Online]. 2011;8:1-3.



