Presentation
A 47-year-old white female reported a one-year history of decreased vision, pain, and photophobia in the left eye. The right eye was asymptomatic. She was evaluated at an outside hospital and diagnosed with presumed non-necrotizing anterior scleritis and anterior uveitis of the left eye. She was referred to the Wills Eye Hospital Uveitis Clinic for further management.
History
Past medical history was notable for iron deficiency anemia and hypothyroidism. She denied history of autoimmune conditions, although prior work-up did demonstrate an elevated p-ANCA. Her surgical history was notable for tonsillectomy in 1996 and Cesarean section in 2003. She had never smoked, nor did she drink alcohol. She had received two Moderna COVID vaccinations. She was taking prednisone 40 mg daily by mouth and methotrexate 15 mg weekly by mouth, supplemented with folic acid 1 mg daily by mouth.
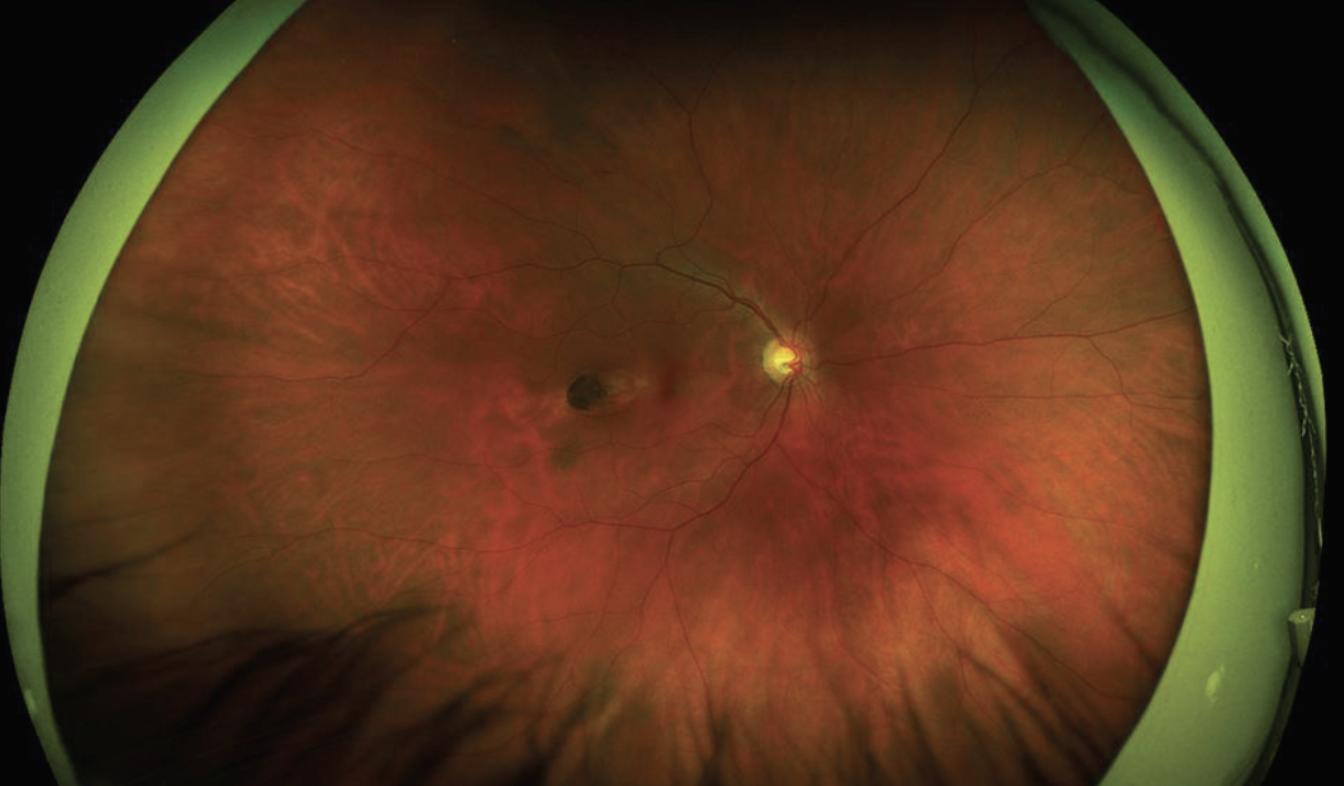 |
| Figure 1. Optos imaging of the right fundus showed an ovoid lesion oriented obliquely towards the foveola. This lesion was located temporally in the macula. |
Examination
Upon presentation to the WEH Uveitis Service, visual acuity was 20/20 in the right eye, and hand motion in the left eye. The right pupil was round, brisk and reactive, and the left pupil was irregular and non-reactive. Extraocular motility was full in both eyes. Confrontational visual fields were full OD and restricted OS with superior and inferotemporal loss. Intraocular pressure was 14 mmHg and 8 mmHg OD and OS, respectively.
Anterior examination of the right eye was normal. The left eye was found to have scleral thickening with superonasal tenderness and conjunctival hyperemia. There was diffuse corneal stromal thickening and haze temporally, with deep stromal haze and vascularization from 9 o’clock to 1 o’clock. The anterior chamber was deep with no cell or flare. There were 360 degrees of posterior synechiae, and a white cataract was present.
Dilated examination OD showed clear media, sharp disc margins with a cup-to-disc ratio of 0.6, and an oval, well-demarcated atrophic chorioretinal scar in the temporal macula measuring about 1.5 x 0.5 disc diameters, oriented towards the fovea (Figure 1). Dilated examination OS was limited by the white cataract.
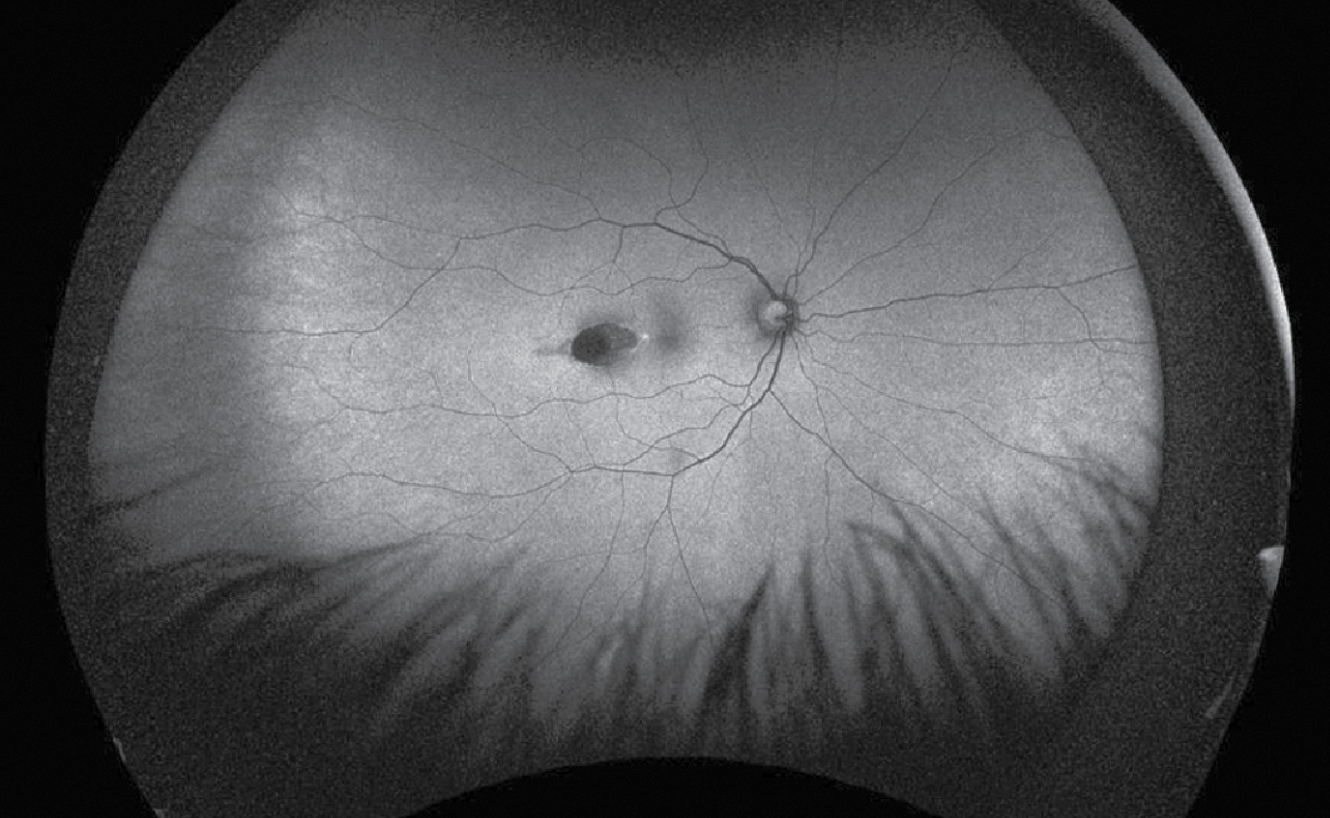 |
| Figure 2. Fundus autofluorescence of the right eye. There was a hyper-autofluorescent, ovoid lesion temporal to the right fovea. |
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
The patient was clinically diagnosed with scleritis with corneal involvement of the left eye. B-scan ultrasonography revealed possible choroidal thickening. The patient was continued on prednisone and methotrexate, in anticipation of transition to infliximab.
Additional retinal imaging was performed on the right eye. Fundus autofluorescence identified a hyper-autofluorescent, ovoid lesion temporal to the right fovea (Figure 2). OCT imaging through the temporal lesion revealed a well-demarcated region of chorioretinal atrophy in the temporal macula pointing toward the fovea with corresponding atrophy and disruption of the EZ/RPE complex; there was no subretinal fluid (Figure 3).
Based on these imaging findings, she was diagnosed with incidental torpedo maculopathy of the right eye. Because the patient was asymptomatic, observation without treatment was deemed appropriate.
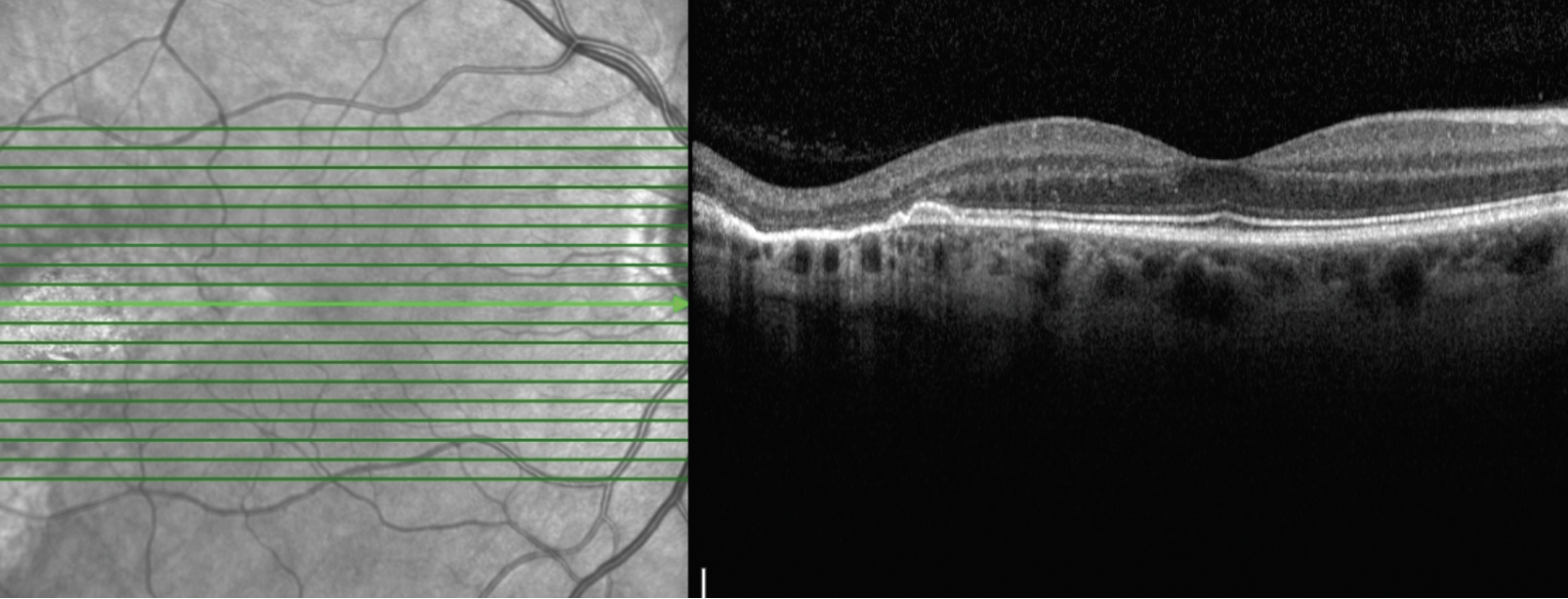 |
| Figure 3. Optical coherence tomography imaging of the right eye through the temporal lesion. There was an oval, well-demarcated chorioretinal atrophy with corresponding atrophy and disruption of the EZ/RPE complex; there was no subretinal fluid. |
Discussion
Torpedo maculopathy—also referred to as solitary hypopigmented nevus of the retinal pigment epithelium, paramacular albinotic spot syndrome or paramacular coloboma—1-4 is a rare, non-vision threatening, RPE and choriocapillaris anomaly. First described in 1971 by Schlernitzauer and Green, and later characterized by Roseman and Gass in 1992, torpedo maculopathy received its apt name in recognition of its ovoid form, which is directed along the temporal raphe toward the foveola.4 One published report within a pediatric population estimated the overall prevalence of torpedo maculopathy at 2 per 100,000, although this prevalence is likely an underestimation, because torpedo maculopathy is often asymptomatic and often presents itself as an incidental finding, as in our patient.5
The cause of torpedo maculopathy remains contested. It’s been hypothesized that torpedo maculopathy arises from aberrant ganglion cell maturation along the horizontal raphe. Other researchers reported that it manifests from persistent defects in the RPE development at the temporal fetal bulge.2,6 It’s also been proposed that the torpedo lesion may arise during the embryologic scleral modification that occurs to accommodate the insertion of the long temporal posterior ciliary artery and nerve, both of which course temporally and anteriorly through the suprachoroidal space and the choroid.7
When found on ophthalmic examination, torpedo maculopathy appears as a unilateral, hypopigmented, torpedo-like lesion located in the temporal retina. In the majority of 10 cases collated by Carol Shields, MD, and her co-authors, the torpedo-like lesion assumed a sharp nasal tip directed within 1 mm proximate to the foveola; the temporal edge sometimes assumed either a rounded or a frayed tail appearance.6 Fluorescein angiography characterization of torpedo lesions indicates a window defect attributable to RPE atrophy.8 OCT-angiography has also been described as a means of detailing RPE atrophy, and has the added benefit of being able to map the underlying choriocapillaris and outer choroidal vasculature.9
Some authors have alleged that different subtypes of torpedo maculopathy exist. One report defined two types of torpedo maculopathy, as detailed by OCT imaging.8 Type 1 torpedo maculopathy is characterized by outer retinal (as defined by interdigitation and ellipsoid zones) attenuation without retinal excavation, whereas Type 2 torpedo maculopathy incorporates outer retinal attenuation and retinal excavation; in both types, the inner retina is intact.
In contrast, Type 3 torpedo maculopathy comprises excavated inner layers, retinal thinning and inner retinal hyper-reflective spaces, with no subretinal cleft.10
Although many cases of torpedo maculopathy are asymptomatic, there are reported cases of clinical significance. Multiple reports have demonstrated that patients with torpedo maculopathy may present with scotomas.7,11 One report hypothesized that these scotomas arise from RPE dysfunction, which in turn leads to improper photoreceptor function.7 Of those cases presenting with scotomas, one of two cases additionally presented with shallow neurosensory serous retinal detachments.11 Additionally, another group reported associated choroidal neovascularization.12
The management of torpedo maculopathy is often limited to observation. For larger lesions, specifically those with “fish-tails,” as described in one study, serial fundus photography and macular threshold perimetry have been suggested.13 In the rare case of associated choroid neovascularization, anti-VEGF treatment was successful.12
Torpedo maculopathy is an eponymous retinal and RPE finding sometimes found on dilated fundus examination. Our case highlights its benign and incidental nature; nonetheless, given reports detailing photoreceptor and RPE atrophy, and in seldom cases, choroidal neovascularization, close observation with fundus photography and macular threshold perimetry may be recommended.
1. Roseman RL, Gass JDM. Solitary hypopigmented nevus of the retinal pigment epithelium in the macula. Archives of Ophthalmology 1992;110:10:1358-1359.
2. Pian D, Ferrucci S, Anderson SF, Wu C. Paramacular coloboma. Optom Vis Sci 2003;80:8:556-563.
3. Schlernitzauer DA, Green WR. Peripheral retinal albinotic spots. Am J Ophthalmol 1971;72:4:729-732.
4. Daily M. Torpedo maculopathy or paramacular spot syndrome. Paper presented at: New Dimensions in Retina Symposium 1993.
5. Shirley K, O’Neill M, Gamble R, Ramsey A, McLoone E. Torpedo maculopathy: Disease spectrum and associated choroidal neovascularisation in a paediatric population. Eye 2018;32:8:1315-1320.
6. Shields CL, Guzman JM, Shapiro MJ, Fogel LE, Shields JA. Torpedo maculopathy at the site of the fetal “bulge”. Archives of Ophthalmology 2010;128:4:499-501.
7. Golchet PR, Jampol LM, Mathura JR, Daily MJ. Torpedo maculopathy. British Journal of Ophthalmology 2010;94:3:302-306.
8. Wong EN, Fraser-Bell S, Hunyor AP, Chen FK. Novel optical coherence tomography classification of torpedo maculopathy. Clinical & Experimental Ophthalmology 2015;43:4:342-348.
9. Ali Z, Shields CL, Jasani K, Aslam TM, Balaskas K. Swept-source optical coherence tomography angiography findings in torpedo maculopathy. Ophthalmic Surg Lasers Imaging Retina 2017;48:11:932-935.
10. Tripathy K, Sarma B, Mazumdar S. Commentary: Inner retinal excavation in torpedo maculopathy and proposed type 3 lesions in optical coherence tomography. Indian J Ophthalmol 2018;66:8:1213-1214.
11. Sanabria MR, Coco RM, Sanchidrian M. OCT findings in torpedo maculopathy. Retin Cases Brief Rep 2008;2:2:109-111.
12. Jurjevic D, Böni C, Barthelmes D, et al. Torpedo maculopathy associated with choroidal neovascularization. Klinische Monatsblatter fur Augenheilkunde 2017;234:4:508-514.
13. Su Y, Gurwood AS. Neurosensory retinal detachment secondary to torpedo maculopathy. Optometry 2010;81:8:405-407.



