Corneal transplants have always been challenging, and outcomes have traditionally been mixed. In recent years however, new lamellar transplants that replace only the anterior or posterior cornea have begun to gain popularity, improving outcomes.
Today, the advent of femtosecond laser technology has generated a whole new set of techniques for performing both penetrating and lamellar procedures. Using the programmable laser to make some or all of the incisions in the donor and recipient corneas makes it possible to achieve almost perfect matches between donor and recipient; allows the donor cuts to be made at the eye bank to the surgeon's specifications and sent to the surgeon pre-cut; ensures greater accuracy in terms of incision depth and location; allows graft edges to be shaped in ways that enhance fit and healing; and makes it possible to create useful additions such as perfectly centered radial alignment marks.
At the same time, this technology has created new practical problems. Most notably, many surgeons can only access a femtosecond laser in a refractive surgery suite, which therefore requires creating the recipient's incisions in one location and moving the patient to an operating room in another location. This has resulted in the strategy of creating incomplete incisions—another feat easily accomplished by the laser—which prevent the anterior chamber from collapsing while the patient is moved to the OR. There, the incisions can be completed manually.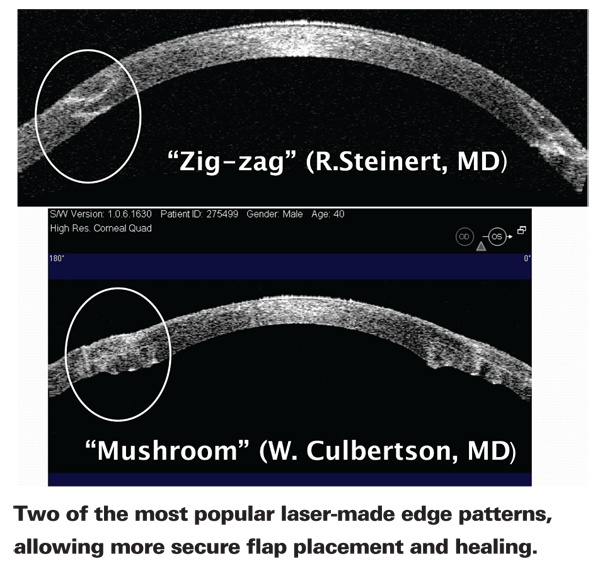
Here, several surgeons with extensive experience performing these laser-enabled procedures share what they've learned and where they see this technology going in the future.
On the Cutting Edge
Unquestionably, one of the advantages of laser-cut incisions is the ability to create a shaped edge using multiple horizontal, vertical and/or diagonal cuts, potentially causing more precise interlocking of graft and recipient and better healing. Currently, three edge designs are in common usage: The top-hat, in which the posterior portion of the graft has a wider diameter than the anterior portion; the mushroom (or inverted top-hat), in which the anterior portion has a greater diameter than the posterior; and the zigzag, in which the edge ends up with multiple ridges running along it.
"We've been using the femtosecond laser for more than two years to perform penetrating keratoplasty for various disorders," says William W. Culbertson, MD, director of the
"The overlapping configuration of the side cut makes the healed incision stronger and more resistant to injury," he continues. "The overlapping edges create better apposition, where the internal pressure of the eye is pushing the tissue together rather than pushing it apart. And you get a more complete seal because you have more surface area healing together than you do with a straight vertical cut. In addition, cutting the incision with the femtosecond laser seems to stimulate healing. At this point we don't have enough data to prove it, but our experience supports these conclusions."
Dr. Culbertson adds that he has treated a few patients with corneal scars using both the mushroom and top-hat edge configurations, and a couple of patients with corneal edema using the zigzag and top-hat edge configurations. "I've stayed with the mushroom shape for keratoconus," he says. "I just vary the dimensions of the mushroom a bit from patient to patient to customize it to the patient's problem and cornea."
Roger F. Steinert, MD, vice chairman of clinical ophthalmology, director of refractive, cornea and cataract surgery, and professor of ophthalmology and biomedical engineering at the 
Francis W. Price Jr., president of the Price Vision Group in Indianapolis and founder and board president of the Cornea Research Foundation of America, says that he's done about 60 femtosecond-laser-assisted transplants. He says he now primarily uses the zigzag pattern. "The zigzag is the most versatile incision," he says. "It interlocks when you put the pieces together, and the surface contour is better. By comparison, the top-hat and mushroom are difficult to suture; if the sutures are too tight the graft buckles; too loose and your wound's not all that great. The zigzag makes these surgeries go much more smoothly. It's also helped to speed healing; we seem to be removing the sutures a lot sooner."
Anterior Lamellar Procedures
The femtosecond laser offers clear advantages when the objective is to remove and replace only the anterior portion of the cornea, thereby avoiding endothelium rejection. "The femtosecond laser is definitely useful with this technique because it gives you control over the dimensions—although no one yet knows exactly what dimensions will turn out to be most effective," observes Perry S. Binder, MS, MD, who practices at the Gordon Binder Vision Institute in San Diego and is co-medical director of AMO/IntraLase. "In lamellar, or partial thickness transplants, the laser makes a horizontal incision in the donor cornea. For anterior lamellar procedures the IntraLase is currently limited to cutting at depths not to exceed 200 µm, because when you go deeper, the visual quality is not as good." He notes that although only the IntraLase and Femtec lasers currently include software for creating posterior lamellar cuts, all femtosecond lasers are currently able to do anterior lamellar procedures.
Dr. Price is working to improve the deep anterior lamellar keratoplasty "big bubble" technique, in which he injects air under the cornea and removes only the anterior tissue, leaving Descemet's membrane intact (assuming that the patient has good endothelium). The donor tissue is placed on top of it to treat corneal scarring, ectasia or keratoconus. "In this technique we use the same femtosecond laser incisions on the patient that we'd use for a penetrating graft, but we don't go all the way into the anterior chamber," he explains. "Because we're not worried about rejection, we can use larger diameters. I think this procedure is going to be much more widely used than penetrating grafts." Dr. Price says he expects to publish clinical data on this work shortly.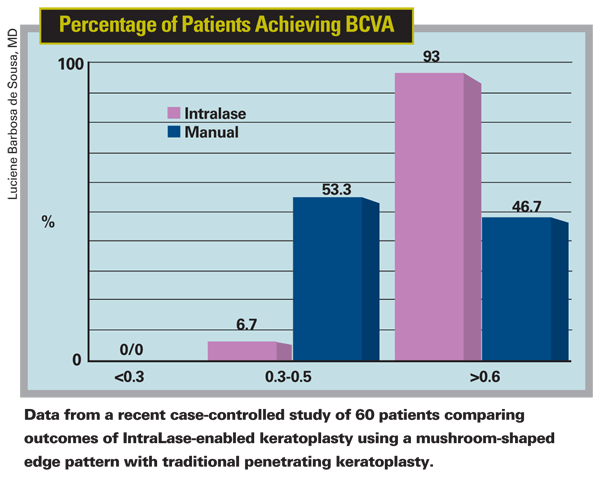
Dr. Binder notes that it's possible to put a shaped edge on a simple anterior graft as well. "It's not really necessary because you're dealing with a very thin flap," he says. "However, with the fifth-generation 150-kHz IntraLase you now can turn the edge bevel in, allowing you to literally tuck the graft edge into the cornea. With that configuration it may be possible to attach grafts with no stitches at all."
Dr. Steinert's team has also been using the zigzag incision to perform DALK with the big bubble technique. They've discovered that this provides an unexpected benefit if a problem arises during surgery. (Dr. Steinert credits his associate
"It turns out that that is just about perfect for the big bubble technique, because the zigzag laser incision is down very close to Descemet's membrane but not through it," he continues. "So if we're performing big-bubble DALK and Descemet's membrane ruptures, we still have a donor with a zigzag incision and a patient with a zigzag incision; we can easily convert from a DALK to a full-thickness PK. We just don't remove the endothelium from the donor button. Either way the patient gets the benefit of the zigzag incision from both the healing and optical point of view."
Cutting Posterior Discs
Endothelial keratoplasty has become increasingly popular as an alternative to penetrating keratoplasty; according to Dr. Price, about one-third of the corneal transplants in the United States are now of this type. However, because the lamellar cuts in the donor tissue are posterior cuts, the issues with using the laser are somewhat different.
Dr. Binder says that when a procedure involves a posterior corneal graft (i.e., DSEK or deep lamellar endothelial keratoplasty), the laser can be used to create the donor graft just as a mechanical microkeratome would, except the shape, edges and thickness are under the surgeon's control. However, the surface produced is rougher at that depth than the surface of a more anterior cut, such as a LASIK flap. "This is also true if you cut at that depth with a mechanical microkeratome," he notes, "because the posterior corneal anatomy is more loosely packed and therefore dissects differently than the more compact anterior stroma. The benefit over mechanical techniques is that you can cut predictably very close to Descemet's membrane, obtaining very thin grafts that are easily rolled and implanted. With a mechanical microkeratome you never know how much stroma you'll have attached to the cornea."
Dr. Culbertson says that so far he doesn't use the femtosecond laser regularly to cut donor tissue for DSAEK because of the less smooth interface. "This doesn't matter when you're making vertical side cuts for penetrating keratoplasty because you're away from the visual axis," he says. "In fact, if it's a little rougher, I think it heals better. But when you're making a post-lamellar button, you're cutting through the middle of the visual axis. 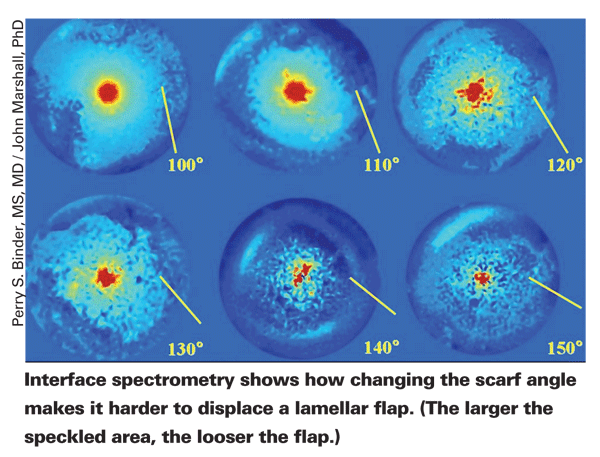
"Also," he continues, "the surface of the donor tissue is applanated by the IntraLase when it makes the cut, and that may affect the surface smoothness of deep cuts by creating circular deep folds." He notes that the Visumax femtosecond laser from Zeiss uses a curved contact plate which could turn out to make a difference by avoiding this particular problem; at present, however, the Visumax laser can't be programmed to make deep enough incisions to cut donor DSAEK lenticules. In the meantime, Dr. Culbertson's colleague, Sonia H. Yoo, MD, has been working with altering speed, energy, spot/line separations and cutting patterns of the IntraLase to try to improve the bed produced when the cut is deeper in the tissue.
Rudy M. Nuijts, MD, PhD, associate professor of ophthalmology at the
"Our approach to femtosecond laser-assisted Descemet's stripping endothelial keratoplasty is to have the eye bank use the laser to create a lamellar interface in the donor cornea at a maximum depth of 400 µm and a width of about 9.5 mm, with no vertical edge cut," he explains. "After tissue preparation and storage the cornea is sent to the surgeon. We can then trephine the cornea to create the desired diameter disc and split the anterior and posterior sections of the cornea, placing the posterior disc—which is about 110 µm thick on average—into the recipient eye. This is far easier than using a microkeratome to make the disc in your clinic."
Dr. Nuijts notes that donor corneas in Europe are maintained by eye banks using a technique significantly different from the "cold storage" technique used in the
Dr. Nuijts says that his group is running a randomized clinical trial comparing the outcomes of posterior lamellar keratoplasty performed with the femtosecond laser to outcomes from conventional penetrating keratoplasty. "So far, astigmatism following posterior lamellar keratoplasty with the laser is pretty low, as you would expect from the results of other posterior lamellar keratoplasty techniques," he says. "But the visual acuity results are somewhat poorer than those from penetrating keratoplasty, and also a little poorer than when the posterior graft is prepared using a microkeratome—about one line lower, on average." (In a study of 20 patients receiving femtosecond laser-assisted DSEK conducted by Dr. Nuijts, average BSCVA improved from 20/110 to 20/57 at six months follow-up. Previous series of DSAEK patients in the literature reported BSCVA averaging between 20/45 and 20/34 at three and six months, respectively.)1-4
"We're working on determining the reason for the difference," he notes. "I'm inclined to believe that the small comparative difference in visual acuity is caused by the wound-healing response at the interface rather than the roughness of the surface."
Challenging Questions
Because these laser procedures are so new, with little clinical data in the literature to support specific approaches, many issues are still being debated. For example:
• What's the best way to prevent rotation and torque? Suturing a round disc in place is potentially problematic; if the sutures are not exactly the same all the way around, they can create torque, leading to astigmatism.
Several novel approaches to creating the laser incisions are attempting to solve this problem. Some companies are modifying the edge of grafts as a way to prevent rotation. "One laser can create 'positional spikes'—little triangles that stick out of the donor graft and fit into matching spaces in the recipient," notes Dr. Binder. "Another approach involves using eight- or 10-sided donor buttons and recipient incisions. Whether these approaches will solve the astigmatism issue remains to be seen; other factors may contribute to the problem, including wound healing issues, suture tension and suture placement."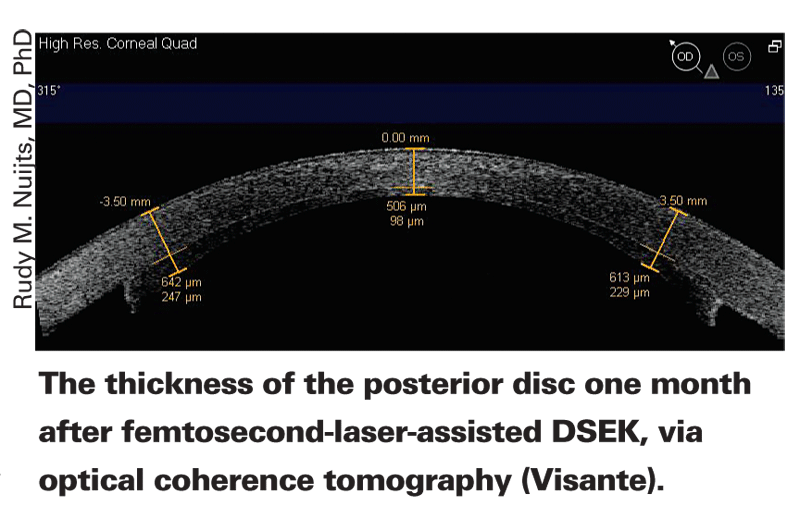
"We've been using radial alignment marks that can be added with the IntraLase," says Dr. Steinert. "The concept of radial alignment marks has been around for a long time; the problem was that there was no way to get marks that are geometrically perfect on both donor and host—especially the donor. If you pre-mark the donor and then punch it, the trephination is not likely to be exactly centered on the radial marks. If you follow off-center marks, you end up with a problem.
"The laser solves that because any markings that the laser makes are guaranteed to be perfectly centered," he says. "Now we routinely have the eye bank place 16 radial alignment marks on the donor following my specifications, and we place eight marks on the patient periphery for alignment. The donor marks also have a specific length so they can act as a guide for suturing. This helps the surgeon be consistent about where the needle enters enter the donor cornea, so the sutures are very symmetrical over 360 degrees. The result is reduced postop visual distortion and no issues with healing because there are no sharp discontinuities."
• If a cone is decentered, should the graft be? An important issue when creating a penetrating graft is having sufficient thickness in the recipient's corneal tissue where the incisions are made. (Most of the surgeons interviewed for this article rely on the Visante anterior segment OCT or the Pentacam to assess this before surgery.) Since the cornea may be thinner closer to the cone, does it make sense to shift the graft when a cone is off-center?
Dr. Nuijts believes it's very important to center the applanation cone over the geometric center of the cornea. "Most of the time, keratoconus is decentered inferiorly," he admits. "However, I always aim to center the applanation cone on the center of the pupil, even if the cone is slightly off-center. Otherwise, you risk having the peripheral incision too close to the chamber angle. I believe the way to make sure the cornea you incise is thick enough is to modify the diameter of your trephination, not to intentionally decenter. Decentering is a good way to generate astigmatism."
Other surgeons expressed less concern about decentering; Dr. Culbertson says that he has decentered inferiorly by as much as a millimeter when a patient has a slightly decentered cone. Dr. Steinert says that because keratoconus patients have large corneas, his approach is to create a large 9-mm incision with a zigzag edge. "That leaves enough tissue to work with that we can get outside the cone inferiorly and decenter downward a little bit if we need to," he says. "Centration isn't the main issue, per se. You just don't want the patient looking through the portion of the transplant that's close to the incision; there's too much distortion in the periphery. But with a large enough transplant, in our experience, slight decentering hasn't caused a problem."
• Should the graft be oversized? Dr. Nuijts says that in the
Here in the
Where Do We Go from Here?
"Using the laser to perform these procedures is a pleasure, and clearly it can achieve accuracy that manual procedures can't," observes Dr. Nuijts. "On the other hand, my own penetrating keratoplasty results and the limited data in the literature indicate that even with the laser we're not reducing induced astigmatism by that much. Clearly we're moving in the right direction, but there are so many variables to consider that it's a challenge."
"I think femtosecond-laser-assisted transplantation has the potential to become the accepted standard procedure," says Dr. Steinert. "We need more data, more people doing it and more long-term experience to demonstrate unequivocally that it's a better procedure, but I think that will happen. Hopefully, it will then broaden our horizons to the next level, which is finding better ways to do the wound closure."
Wherever this technology leads next, Dr. Nuijts says he's convinced that it's necessary if corneal surgeons want to make their results more predictable, and he's happy to be doing this work. "This is still early progress, but it's very innovative," he says. "Using femtosecond lasers in corneal transplantation is one of the major developments in corneal surgery in the past two or three decades, and one of the most exciting things I've been involved with."
Drs. Culbertson and Price are consultants to AMO/IntraLase Corp.
1. Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25:8:886-889.
2. Koenig SB, Covert DJ. Early results of small-incision Descemet's stripping and automated endothelial keratoplasty. Ophthalmology. 2007;114:2:221-226.
3. Koenig SB, Covert DJ, Dupps WJ, Jr., Meisler DM. Visual acuity, refractive error, and endothelial cell density six months after Descemet stripping and automated endothelial keratoplasty (DSAEK). Cornea. 2007;26:6:670-674.
4. Covert DJ, Koenig SB. New triple procedure: Descemet's stripping and automated endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation. Ophthalmology. 2007;114:7:1272-1277.
5. Farid M, Kim M, Steinert RF. Results of penetrating keratoplasty performed with a femtosecond laser zigzag incision initial report. Ophthalmology 2007;114:12:2208-12.