Although the primary concern in most glaucomas is elevated intraocular pressure caused by insufficient outflow of aqueous, correcting that problem can be difficult. As a result, many of our current treatment options attempt to compensate for outflow insufficiency by reducing aqueous production.
One way to do that is to ablate a portion of the ciliary processes that produce the aqueous. Lasers, with their ability to treat a focal area, provide a practical way to accomplish this. When lasers were first used for this purpose, the application probe was positioned against the sclera overlying the processes—i.e., transscleral cyclophotocoagulation (TCP). In recent years, the development of endoscopic technology has allowed us to move the application of the laser light, along with a video portal, inside the eye, to perform endoscopic cyclophotocoagulation (ECP).
ECP vs. TCP
Both forms of cyclophotocoagulation have advantages and disadvantages, which determine their differing indications. The main advantage of TCP is that it's nonpenetrating. However, TCP is usually done without being able to directly visualize the target tissue (although some surgeons do use transillumination to permit some visualization). The result is that it's easy to overtreat, leading to more serious complications. When treating, many surgeons titrate to a popping sound that indicates an effect has been achieved, but this sound usually means that tissue has exploded and you've overtreated.
In contrast, ECP allows you to look directly at the ciliary processes as you're treating them. This makes it possible to be much more selective about which tissues you treat, and lets you titrate the energy to avoid overtreatment. Both of these factors result in a reduced likelihood of side effects and complications. However, ECP is an intraocular procedure, so you have to take the patient to the OR and create an opening in the eye similar in size to that for phacoemulsification. This creates other risks not present when a procedure is nonpenetrating, such as infection and choroidal hemorrhage.
As a result, TCP and ECP are appropriate for very different situations. Because TCP is noninvasive but may result in more side effects, it's usually reserved for eyes with poor visual potential that are difficult to treat. In these cases, TCP would be used if other procedures and medications had failed. On the other hand, ECP may make more sense when the eye is already opened for another surgery such as cataract removal, because the risks associated with opening the eye are already present. Typically ECP is performed in eyes that have relatively good visual potential, to justify intraocular surgery.
A Paradigm Shift
Because it destroys tissue and can result in significant complications, ophthalmologists have traditionally thought of cyclophotocoagulation as a surgery of last resort, something to be done after first trying maximal medications and a filtering procedure. Recently, however, this attitude has begun to change. Several papers have reported on cyclophotocoagulation as a primary surgical treatment, or even as an overall first-line treatment as in one study done in
Part of the reason for this shift is that recent studies have found ECP to be safer than previously thought; there doesn't appear to be a significant risk for many of the complications traditionally associated with transscleral cyclophotocoagulation such as hypotony, phthisis and vision loss. As the risk of complications grows smaller, cyclophotocoagulation becomes a more reasonable option for use early in treatment.
One reason ECP may pose less risk than TCP is that it doesn't eliminate all of the ciliary processes. Typically, we can only access limited portions of each ciliary body; the areas between the processes also make fluid, and sometimes you can't treat those areas.
In a recent study from my lab group published last year in the British Journal of Ophthalmology2 we used an animal model to look at the histology as well as vascular perfusion after TCP and ECP. We found that TCP caused significant, long-lasting obstruction to the blood flow of the ciliary processes. In contrast, ECP caused an initial reduction in blood flow, but there was a partial return of blood flow after one week and even more after one month. The fact that blood flow is not completely cut off following ECP may explain why there appears to be significantly less risk for hypotony or phthisis with this procedure. In essence, it maintains some of the health of the ciliary processes.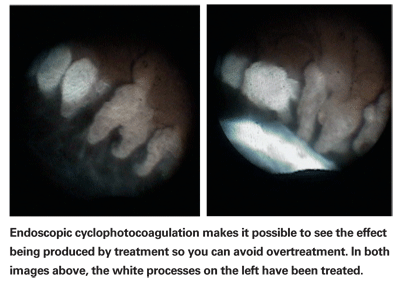
Despite this advantage, I would caution against the extensive use of ECP as a first-line treatment, partly based on the data from a study we conducted at the University of California, San Francisco, several years ago.3 In that study we treated 68 eyes of 68 patients with ECP, including patients with various types of glaucoma; diagnoses included primary open-angle, congenital, chronic angle-closure and neovascular glaucoma. Although the treatment was effective, we encountered several types of complications (the majority of which were not severe) ranging from inflammation inside the eye and fibrin formation to cases of transient hyphema. The complications that were more visually significant mainly involved cases of cystoid macular edema; in our series of patients, the risk of CME was 10 percent. Thus, in patients who are at greater risk of macular edema (e.g., diabetic and uveitic patients) ECP should perhaps not be considered as a first-line surgical option.
ECP and Cataract Removal
Currently, ECP is most often used in conjunction with cataract extraction, for the reason mentioned earlier: In this situation you're already taking the patient to surgery and opening the eye. ECP gives you the opportunity to do a laser procedure that's relatively simple and appears to have relatively few complications, as a way of reducing high pressure or decreasing the number of glaucoma medications the patient needs to use.
One recent study by Stanley Berke, MD, of
However, combining ECP with cataract surgery would not be the ideal choice in every situation. It appears to be a sensible procedure if a patient's pressure is controlled and reducing medications is the goal. However, the drop in pressure produced by ECP is modest; the average IOP reduction was about 10 mmHg in the study at UCSF.3 For that reason, I wouldn't recommend ECP in eyes with advanced glaucoma and very elevated IOP. If a cataract patient is on three medications and I don't think the pressure is controlled well enough, I would probably combine phaco with a trabeculectomy instead.
Practical Pearls
If you're considering using TCP or ECP as a part of your glaucoma armamentarium, here are a few strategies to help maximize your outcomes:
• Be careful at the outset. Like any surgery, this has a learning curve; get training before doing it in patients. There have been reports of severe complications, including one in which a surgeon accidentally caught the ECP probe on the iris and pulled the entire iris out of the eye.
• Use viscoelastic. I inject an ample amount of viscoelastic to inflate the ciliary sulcus. This pushes the iris anteriorly, giving you better access to the ciliary processes. You can visualize them more clearly and do a better job of treating them, with maximum access. In this situation, I recommend using a cohesive viscoelastic. If you use a high-viscosity viscoelastic, it's important to remove it completely at the end of the case to prevent postoperative IOP elevation.
•Treat at least 270 degrees. The data from our original study suggested strongly that treating 90 or 180 degrees was not as effective as treating 270 degrees, which is what we ultimately recommended.
I'm often asked whether I have a treatment formula; i.e., "This patient is using this many medications and her pressure is 35, therefore I'm going to treat this number of degrees." The answer is no; I generally treat all adults with 270 degrees or more. So far, severe complications such as severe hypotony or phthisis have not been reported from ECP in adults, and our vascular perfusion study demonstrated that ECP doesn't completely shut down aqueous production.
• Consider a curved probe. Endo Optiks, the company that manufactures the laser, now offers curved endoscopic probes. If you prefer to use only a single cataract incision when combining ECP with phaco, these may allow you to treat up to 270 degrees of the ciliary processes instead of only 180.
• Treat the whole process. Try to treat both the anterior and posterior extents of the ciliary processes; if you just treat the tips, you may be missing 50 percent or more of the tissue that could be producing aqueous.
• Don't overtreat. The correct titration for the ECP is that you see shrinkage and whitening of the ciliary processes on the video screen when you treat them. If you see a bubble form on the tissue—i.e., the tissue has exploded—you've put too much energy at that spot, either because the energy level is set too high, or you left the probe in one spot for too long. You may also need to be a little farther away from the processes to avoid concentrating the energy on a smaller area. Overtreatment can lead to greater inflammation/fibrin formation, cystoid macular edema, and hyphema.
• Avoid possible drug interactions. So far, there's no clinical data regarding whether TCP or ECP combine better (or worse) with any particular medications. However, our study indicated a 10-percent risk of CME after ECP. Since the likelihood of CME may increase after ECP, patients who are on prostaglandin analogues may be more at risk. Therefore, if you're considering reducing a medication early on as a result of lower pressure, you might consider reducing that one.
• Consider using OCT to check for CME. Since most cases of CME are not clinically significant, this condition may be missed. Unfortunately, low-level CME after ECP can mean a serious risk for later vision loss, particularly if a patient needs to start using a prostaglandin. For that reason, careful observation of the macula under stereoscopic conditions is recommended. OCT technology is another simple, safe and noninvasive way to evaluate the eye's condition.
Outlook: Positive
The amount of research being done on ECP has increased noticeably in recent years, as ophthalmologists realize that the procedure is relatively safe. Hopefully, future studies will provide longer-term follow-up on such patients and more information about the possibility of CME and other vision-threatening risks.
Dr. Lin is associate professor of clinical ophthalmology at the
1. Egbert PR, Fiadoyor S, Budenz DL, Dadzie P, Byrd S. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol. 2001;119;3:345-50.
2. Lin SC, Chen MJ, Lin MS, Howes E, Stamper RL. Vascular effects on ciliary tissue from endoscopic versus trans-scleral cyclophotocoagulation. Br J Ophthalmol. 2006;90:496-500.
3. Chen J, Cohn RA, Lin SC, Cortes AE, Alvarado JA. Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol. 1997;124:6:787-96.