A key turning point in the evolution of biometry occurred with the switch from ultrasound measurement to optical biometry. “The advent of refractive cataract surgery really began with the use of optical biometry, starting with the first IOLMaster more than a decade ago,” notes Eric D. Donnenfeld, MD, a clinical professor of ophthalmology at New York University Medical Center and a partner at Ophthalmic Consultants of Long Island. “For the first time, we had the ability to more accurately predict IOL power in patients having cataract surgery.” Since that development, a host of additional technological improvements have pushed the envelope even further, steadily increasing the accuracy and reproducibility of modern biometry devices.
With ever-more-powerful tools at ophthalmologists’ disposal, the importance of using them effectively to create optimal outcomes increases every year. Here, ophthalmologists with extensive experience in optical biometry discuss how the technology has evolved in recent years, and share their advice for making the most of today’s instruments.
New vs. Old Technology
Today’s biometry devices serve today’s ophthalmologists better than the older instruments, for a number of reasons. One reason is the increasing number of patients who‘ve undergone previous refractive surgery. “It used to be that the person who had prior refractive surgery was sort of a curiosity—an unusual situation for a cataract surgeon to encounter,” says Terrence P. O’Brien, MD, a professor of ophthalmology and director of the Refractive Surgery Service at Bascom Palmer Eye Institute of the Palm Beaches. “I can recall when we only saw a few cases. They’d throw our routine off; we’d have to step back and perform a lot of extra calculations and try to figure everything out. Now, people who’ve had prior refractive surgery of one type or another are presenting routinely every week.
“This is where some of those bells and whistles on the new devices are valuable,” he continues. “Despite all of the changes we’ve seen, axial length and keratometry still have to be determined accurately, and thanks to today’s technology, axial-length measurement has come a long way. That process used to involve a one-dimensional A-scan ultrasound; it was subject to a lot of variability that could have a significant impact on the outcome. The patient wasn’t obliged to fixate or look in one direction, but if the patient wasn’t properly aligned the measurement might not represent the actual distance to the fovea. That was a big problem.
“Of course, even back then there were ways to try to determine whether the measurement was accurate,” he says. “You could examine the peaks on the printout, and with some experience you could tell if the patient was looking askew, meaning you weren’t getting reliable data. But today, determining the axial length optically
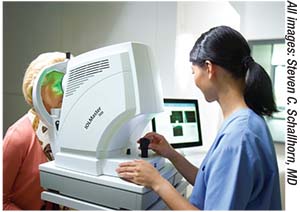 |
| Today’s biometry devices perform multiple functions with minimal technician management, but technicians still need thorough training to catch warning signs that a reading isn’t reliable. |
Dr. O’Brien adds that these instruments also have improved the reliability of keratometry measurements. “That’s the other key variable for predicting the optimal lens power,” he notes. “It’s a challenge to obtain these measurements, because the measurement process isn’t the only issue; the ocular surface may change over time. That means obtaining good keratometry may require repeat measurements to make sure the ocular surface is stable. Also, when manual keratometry was the gold standard for measuring corneal power, user skill and experience were very important. If you didn’t make the measurements yourself, you weren’t always sure of the quality and accuracy of the data. Today, automated keratometry is built into the popular optical biometers, so that process is now systematized into a single device and the data is obtainable with a single click in most cases. That increases accuracy and makes it easy to obtain repeat measurements.”
Do You Need the Very Latest?
Given the rapid advances in biometry technology and frequent addition of new features, surgeons today are faced with the question of when it’s worth upgrading to the “latest and greatest” iteration.
“All of the modern optical biometers are good when it comes to measuring axial length,” says Dr. Donnenfeld. “What the newest machines do, particularly the IOLMaster 700, is use swept-source OCT to verify the location of the macula. That helps prevent axial-length measurement errors. In fact, errors in the keratometry, rather than the axial-length measurement, have become the number-one reason patients have erroneous IOL power predictions. But even the keratometry is more reproducible because the IOLMaster 700 uses telecentric keratometry. That technology makes the measurements more reproducible even when the user doesn’t have the keratometry in perfect focus.”
“The latest versions of the IOLMaster and Lenstar are really terrific,” agrees Dr. O’Brien. “They allow us to measure additional parameters, including lens thickness, corneal pachymetry and anterior chamber depth. And now several of the latest instruments, like the IOLMaster 700 and some competitors, have added swept-source optical coherence tomography. They acquire the measurements even more quickly, and even with a very dense cataract we can obtain a reliable measurement with a two-dimensional configuration of the foveal anatomy.”
Steven C. Schallhorn, MD, former director of cornea and refractive surgery at the Naval Medical Center in San Diego, now in private practice in San Diego, believes the latest generation of biometers will improve the predictability of outcomes. “It’s not that outcomes were unsatisfactory with earlier-generation biometers,” he says. “This is simply an evolutionary step forward. The latest-generation devices provide measurements for the most advanced power calculation formulas, and they make it easier to ensure the accuracy of their measurements. Having more accurate measurements and added parameters for certain formulas will collectively drive better outcomes.”
Dr. Schallhorn notes that historically, surgeons relied on simple keratometry, which measures only two points on the cornea. “Basic keratometry makes a number of assumptions about the corneal shape, such as that the cornea is spherical (which it isn’t), and it applies a simplistic adjustment for the power of the posterior cornea,” he points out. “Those assumptions have been considered reasonable for the majority of patients, but they are not reasonable, for example, in patients who’ve had prior refractive surgery. Corneal asphericity and other anomalies can significantly impact proper IOL power selection. Biometers that derive more information from the cornea are going to help improve outcomes.”
Dr. Donnenfeld agrees, but with a qualification. “The more advanced technologies give patients a better chance of achieving the spectacle independence they’re looking for,” he says. “Every time you have an improvement in technology there’s an incremental improvement in the quality of the measurements, and we’ll continue to see incremental improvements as the technologies get better over the next couple of years. But these are incremental improvements, not disruptive ones. The older technologies are still very good, and the delta is not that great. I think the most important factor is using optical biometry instead of A-scan ultrasonography.
“As we all know, better technology generally yields better results, so it’s nice to have the latest technology,” he adds. “In addition, the new technology has dramatically reduced the amount of time required to take these tests. The new IOLMaster 700, for example, takes less than 30 seconds to perform a reading. However, at the practical level it’s always a cost-effectiveness decision the surgeon has to make.”
Before Taking the Measurements
To help ensure the most accurate and reproducible biometry data, surgeons recommend these strategies:
• Make sure your technicians are well-trained. The latest instruments have definitely reduced the likelihood of operator error, but errors still can (and do) happen. A significant factor in preventing errors and correcting them quickly if they occur is having an astute, well-trained technician doing the data capture. “The person you’re trusting to make the biometry measurements should be well-trained and capable,” says Dr. Schallhorn. “You need to be confident that the technician knows what he or she is doing and can obtain a proper capture.”
How much t
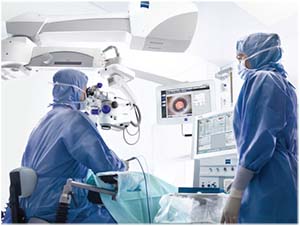 |
| Surgeons should always take the time to double-check the biometry data for tell-tale warning signs of an unreliable reading before proceeding with surgery. |
• Rely on just a few technicians to acquire your biometry measurements. “This is not a situation in which you want to cross-train dozens of people,” says Dr. O’Brien. “It’s better to have one or two designated individuals in your practice who do this repeatedly. When someone does this over and over, they’re more likely to know when the measurement needs to be redone; they’re less likely to miss something that violates the validation criteria. That means you’ll be getting better reliability and higher reproducibility. Having five or six people sharing this task will introduce more variability into your practice. Of course, you might want to cross-train a few technicians in case of illness or absence, but it’s better to have just a couple of ‘go-to’ people who do this over and over again. This will provide more reliable, reproducible data.”
• Be sure to ask whether the patient wears contact lenses. “Many patients old enough to have cataracts don’t wear contact lenses, but some do, and that’s critical to note,” Dr. Schallhorn points out. “That can affect the accuracy of your biometry measurements, especially if the contacts are gas permeable.”
Dr. Donnenfeld agrees. “We ask patients to remove their soft contact lenses for at least three days prior to biometry—a week ahead if the soft lenses are toric,” he says. “If the patient wears gas-permeable lenses, we have the patient leave them out for a month so the cornea can return to its normal shape. This helps to ensure that both the biometry and keratometry are accurate.”
Dr. Schallhorn adds that the surgeon should be on the lookout for any patient that has a recent history of orthokeratology. “As with RGP contact lens wearers, those contact lenses can alter the corneal shape for weeks or months after discontinuing.”
• Make sure the ocular surface and tear film are stable. “Because patients are eager to complete their cataract surgery and the team is trying to expedite that process, there may be a little bit of a rush to the OR,” notes Dr. O’Brien. “In reality, some patients may have significant ocular surface disease that should be treated adequately before biometry is repeated. So make certain that the patient’s ocular surface is stable before relying on these measurements.”
 |
“Patients with ocular surface disease have been shown to have markedly reduced accuracy on their keratometry,” Dr. Donnenfeld points out. “For that reason we want to optimize the tear film prior to performing IOL calculations. To that end, we like to use artificial tears, and if necessary, immunomodulation with cyclosporine or lifitegrast. We also believe having the patient take an omega-3 supplement will help to improve the quality of the tear film.”
• Don’t use eye drops of any kind right before taking corneal measurements. Jack T. Holladay, MD, MSEE, FACS, a clinical professor of ophthalmology at Baylor College of Medicine and the developer of the Holladay I, II and Refractive formulas, notes that although the intention when using drops before taking a measurement may be to offset dry eye in the interest of obtaining a more accurate reading, the drops can cause corneal steepening or punctate epithelial keratitis. “A better approach is to simply ask the patient to blink frequently,” he says. “The measurement should be taken about one second after the final blink, which will allow the tear film to stabilize.”
• Postpone biometry in patients with obvious superficial punctate keratitis in the visual axis. “Measurements taken under these conditions will almost certainly be inaccurate,” Dr. Donnenfeld notes.
When Taking the Measurements
These strategies will help prevent inaccurate measurements:
• Make sure your technician is paying attention to patient fixation. “This is a key source of inaccurate measurements,” says Dr. Schallhorn. “If your unit includes OCT with macula-imaging technology, you can check the image of the foveola to confirm fixation. However, most units don’t have OCT yet. That means the technician has to be paying particular attention to this issue.”
“This may be an especially big problem in patients who are highly myopic,” adds Dr. Donnenfeld. “Actually visualizing the biometry location with new OCT biometers such as the IOLMaster 700 gives the surgeon certainty that the biometry is focused in the right place.”
• If you perform auto-K, make sure the keratometries agree. “It’s good to verify that the keratometry found on the optical biometer correlates with the keratometry on auto-Ks or topography, whether you’re using the IOLMaster or the Lenstar,” says Dr. Donnenfeld. “A lot of people verify these results with a different reading. Clinicians in general have stopped using manual keratometry, but many doctors still do autokeratometry and topography.”
Dr. Schallhorn agrees. “If the biometer-derived keratometry is substantially different from manual or topographic keratometry, that’s a sign of trouble,” he says.
• Teach your technician to check the standard deviation on the printout. “The IOLMaster, the Lenstar and the Pentacam AXL all provide a standard deviation reading on their printouts, based on the multiple K-readings they take, usually three,” notes Dr. Holladay. “Doctors usually see standard deviation expressed in diopters, because diopters are meaningful for ophthalmologists at a practical level. They know that a standard deviation greater than 0.2 D indicates a problematic reading, which generally happens in about 30 percent of eyes. In most normal patients with a healthy cornea, that number should be close to zero.
“However, these printouts present this information in millimeters or microns instead of diopters,” he continues. “It’s the same measurement, just expressed in different units. K-readings take a measurement of the anterior corneal radius in millimeters and convert it to a diopter power. For example, a cornea that’s 7.5 mm in radius has 45 D in keratometric power. So a standard deviation of 0.2 D will be presented as the equivalent radius measurement, which is 0.03 mm,
or 30 µm. That means that if the standard deviation on the printout is that amount or greater, it’s exactly the same as a standard deviation of 0.2 D or greater. It’s a warning flag that the measurement can’t be trusted because it’s not repeatable.
“The problem is, if the technician doesn’t understand the significance of this number, he or she will ignore it,” he says. “So, you need to make sure the technician knows about this and checks this number. If the number is high, then the patient should be taken to the topographer or tomographer to confirm the measurement, and the technician should try to determine the reason for the problem. The patient may have dry eye, irregular astigmatism, keratoconus or other issues. By the time the doctor sees the report, the IOL calculation is done; it shows the lens and power. So, the technician needs to be aware of this to catch the problem before the IOL calculation is performed.”
• Make sure the technician checks the signal-to-noise ratio of the axial-length measurement. “If that ratio is less than 2, it’s not a reliable measurement,” says Dr. Holladay. “The ratio can go as high as 12, which is good; if it’s 2 or less, you shouldn’t use that axial length measurement. The technician should go back and redo it. If a good ratio can’t be obtained using that instrument, it may be necessary to use an ultrasound machine to measure the length of the eye. So, be sure your technicians check this number and don’t accept the measurement if the signal-to-noise ratio is below 2.”
• Be on the lookout for disparities between the left and right eyes. “Unless there’s a good reason for the disparity—and there could be—a great disparity between the two eyes is another indication that at least one of the measurements is inaccurate,” says Dr. Schallhorn.
Knowledge is Power
Although it’s easy to simply rely on technology to carry the day, the odds of catching an error go up significantly if you have a solid understanding of what the technology is doing.
“In order to achieve high-quality refractive outcomes, having a basic understanding of the technology and formulas is essential,” says Dr. O’Brien. “That understanding makes it possible to readily determine the quality and reliability of your preoperative data and formula predictions. Fortunately, it’s become increasingly simple to obtain reliable, high-quality measurements using today’s noncontact optical biometers. These devices also have the advantage that many of them come with the most advanced IOL power calculation formulas built right into the platform. Nevertheless, the better you understand what’s happening, the more likely you are to have consistently good outcomes. That’s especially true when you’re dealing with complex cases, such as eyes that fall outside of the average range, or eyes that have had prior surgery—in particular, prior refractive surgery.”
For example, it’s helpful to know that different prior refractive surgeries will affect the corneal radius of curvature differently. “Both the front and back surfaces of the cornea have refractive power, and that has to be accounted for when predicting the optimal lens power to implant,” notes Dr. O’Brien. “For that reason, after you acquire your measurements, an index of refraction is applied to approximate the effective keratometric power of the entire cornea. That’s where you may sometimes encounter problems, because that index of refraction is not the actual refractive index of your patient’s cornea. It’s a number that’s based on the anterior and posterior surfaces in an average eye.
“This complicates matters when an eye has had previous refractive surgery because of the changes in corneal curvature caused by that surgery,” he continues. “If the patient had radial keratotomy, with radial incisions in the periphery to flatten the central cornea, both the anterior and posterior curvatures will have been flattened together. In contrast, PRK and LASIK change the anterior curvature while leaving the posterior curvature relatively unchanged. That means the fudge factor for the radius of curvature is likely to be inaccurate in these eyes. That’s significant because most of the power formulas calculate the total corneal power and the effective lens position based on the anterior surface radius of curvature measurements, so an error in that number can lead to myopic or hyperopic surprises after cataract surgery.”
Dr. O’Brien notes that the latest formulas are getting better at taking this problem into account. “The latest devices and formulas are designed to help determine a more accurate corneal power in these eyes, to account for the keratometric changes following refractive surgery,” he says. “Some of our organizations are also working to help streamline this process for cataract surgeons. The American Society of Cataract and Refractive Surgery has a post-refractive IOL calculator that’s online and accessible, that allows surgeons to use many of these formulas in order to compare and select the optimal power for an individual patient.”
Practical Concerns
A few more strategies can help ensure optimal refractive outcomes:
• Take time to verify for yourself that the readings make sense. “The technician should look at the standard deviation of the axial-length readings to make sure the standard deviations make sense and the numbers are reproducible,” notes Dr. Donnenfeld. “However, it’s always a good thing to have surgeon verification.”
Dr. Schallhorn agrees. “The surgeon is ultimately responsible for ensuring that biometry was done properly and the correct IOL was selected and implanted,” he says. “A good technician will pick up a problem and address it immediately, but the surgeon should double-check the biometry numbers. Is there an unexplained disparity between the left and right eyes? Is there a good correlation between repeated captures? Is there a reasonable match between manual and biometry keratometry? Does the biometry keratometry reasonably match the topography? These are all things that the surgeon should look at to confirm that the calculation is going to be based on an accurate measurement.”
• If you have OCT, consider checking the macula before planning your surgery. “Even if OCT is not built into your biometer, it’s worth performing in a cataract patient,” says Dr. Schallhorn. “It can reveal abnormalities on the macula itself, such as an epiretinal membrane. An epiretinal membrane can be hard to detect clinically, but it’s relatively easy for an OCT to detect. If an epiretinal membrane is partly or mostly responsible for the decrease in vision, that’s very important information to help drive optimal care.”
• Be sure to use the latest correction if the eye measures longer than 25 mm with optical biometry. “Surgeons should be aware that very long eyes produce inaccurate axial- length measurements when measured using current optical biometry,” says Dr. Holladay. “The result tends to be a hyperopic surprise. Doug Koch, MD, figured this out a few years ago and co-developed the Wang-Koch regression formula to correct for the error, to produce a better outcome. However, the formula tends to result in switching the hyperopic error to a smaller myopic error.
“Recently, I was able to analyze this measurement problem using a much larger database—20,000 eyes from Kaiser-Permanente’s database, compared to the 200 eyes Doug Koch worked with,” he continues.1 “That allowed us to run a nonlinear regression. As it turns out, the measurement error becomes progressively greater the longer the eye, so correcting for it with a curve rather than a straight line produces better results; hence the nonlinear regression is more accurate that the linear regression. It results in zero mean error rather than a myopic or hyperopic error.”
Dr. Holladay says that as of November 2017 the new nonlinear regression formula has been implemented in the Holladay IOL Consultant software, so that it can be used with the Holladay I and II formulas. “It’s also available online at hicsoap.com under the calculator tab,” he says. “There are more than 1,000 surgeons out there using our software, but the other 9,000 need to be addressing this problem in long eyes, too. In the meantime, we’ve made the nonlinear regression available to the IOLMaster people so they can modify the formula in the instrument as well.”
• Keep tracking your outcomes. “Despite the advances in the technology that help make the whole process more user-friendly, reliable and accurate, surgeons should not stop tracking clinical outcomes,” advises Dr. O’Brien. “That’s a very important part of this process. The lens constant that’s a component of the IOL calculation formulas should be optimized for the individual surgeon’s outcomes, for each IOL that you might use. Optimization is a way to fine-tune this process and gain even greater accuracy and higher-quality refractive outcomes.”
What Lies Ahead?
The trend toward ever-improving biometric technology shows no sign of stopping, and the upcoming years will bring even more advances. Those advances will include technology that can analyze the posterior corneal surface. “It’s only been within the past several years that we’ve appreciated the role that the posterior cornea plays in IOL power selection,” says Dr. Schallhorn. “We now understand that to better estimate the optical power of the cornea, we need to accurately assess the posterior corneal shape. For example, whether posterior astigmatism is with-the-rule or against-the-rule has an impact on the outcome, and if the patient has had laser vision correction, the difference between the posterior and anterior corneal shape has been altered. That can play a significant role in the IOL power calculation.
“Unfortunately, that technology is not yet available in biometers,” he says. “Right now we have topography devices that can measure the posterior shape of the cornea; that allows us to take the posterior cornea into account in our calculations. However, because the measurement is not made in the biometer itself, it can be time-consuming, costly and inefficient. Next-generation biometers will address this.” REVIEW
Dr. Schallhorn is chief medical officer for Carl Zeiss Meditec. Dr. Donnenfeld is a consultant for Alcon and Zeiss. Dr. Holladay is president of Holladay Consulting, which is the distributor of the Holladay IOL Consultant Software (hicsoap.com). He is a consultant to Carl Zeiss Meditec. Dr. O’Brien has no financial ties to any product mentioned.
1. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology 2018;125:2:169–178.