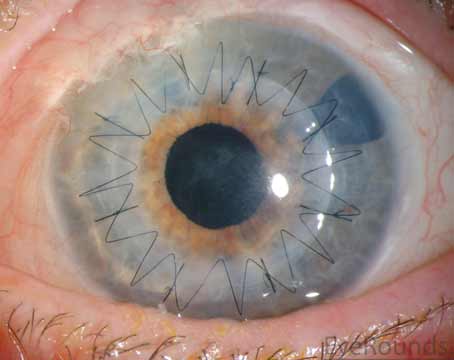
Compared to lamellar keratoplasty, full thickness penetrating keratoplasty inherently has an elevated risk of immunogenic rejection, an issue that’s exacerbated in higher risk clinical scenarios such as herpetic infection, autoimmune disorders and a history of multiple graft failures. In fact, indication for surgery is the most important predictor of graft survival after transplant; for example, keratoconus has been shown to have some of the highest graft survival rates,1–4 while prior infectious keratitis portends a relatively lower graft survival.5,6
The last two decades have seen a shift away from PKP and towards lamellar keratoplasty. However, PKP remains the treatment of choice for combined endothelial and stromal pathology and still accounts for roughly one-third of corneal transplants performed in the United States, including most high-risk keratoplasty.7 Careful preoperative planning, intraoperative considerations and postoperative care can improve surgical success and prolong graft survival. In this article, we discuss our approach to corneal transplant management in complex, high-risk scenarios.
Multiple prior graft failures/Uveitis
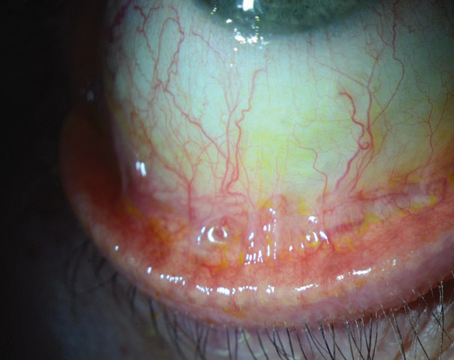
Conditions including uveitis and multiple failed corneal transplants increase the risk of immunogenic rejection. Careful preoperative planning is essential to improve the chances of a successful surgical outcome. The differences in graft survival for each regraft can be stark, decreasing from 43-percent survival for the second regraft, to 25 percent by the third and fourth regraft.8 Although not fully understood, factors such as a reduction in the blood-aqueous barrier9 and neovascularization of the cornea, allow the immune system to become exposed to the foreign tissue.2,10 Given the lower rates of survival, it’s prudent to manage patient expectations, taking care to help them understand the more intensive postoperative care course and the reduced chances of graft survival.
Preoperatively, the surgeon should evaluate and address anatomic features that may accelerate endothelial decompensation. For example, stromal neovascularization leads to incremental increases in graft failure risk.11–13 This is likely due to increased exposure of the immune system to foreign antigens. In light of this relationship, steps should be taken preoperatively to reduce corneal neovascularization. In certain situations, such as following a severe corneal ulcer, delaying surgery can help allow the eye become less inflamed. Preoperative topical steroids, and, potentially topical anti-VEGF therapies, may also be used. Vascular endothelial growth factor (VEGF)-A is a known promoter of neovascularization, and anti-VEGF-A neutralizing antibodies have been shown in experimental models to affect corneal angiogenesis.14–16 In a recent pilot study, subconjunctival bevacizumab was shown to have a positive effect on endothelial rejection, though this wasn’t statistically significant.17 Vigilance should be maintained in the postoperative period, as patients can often require more potent immunosuppression with systemic medications.
 |
|
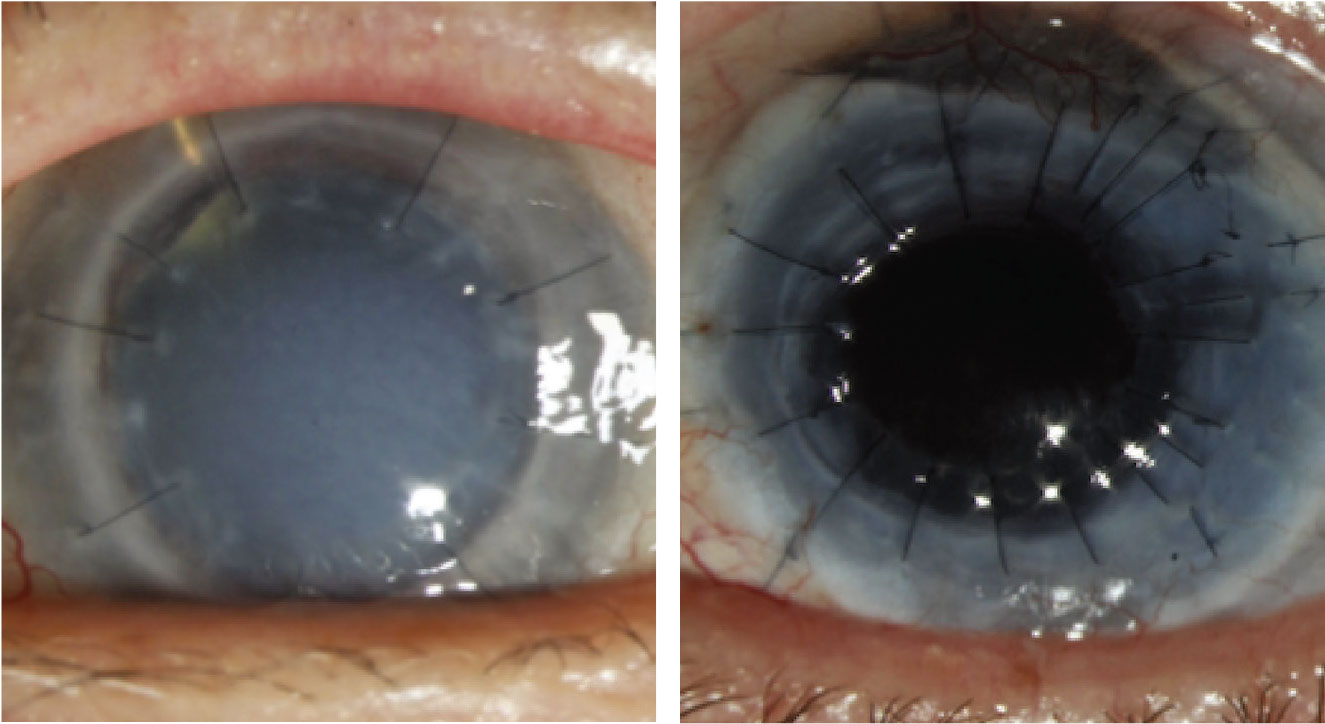
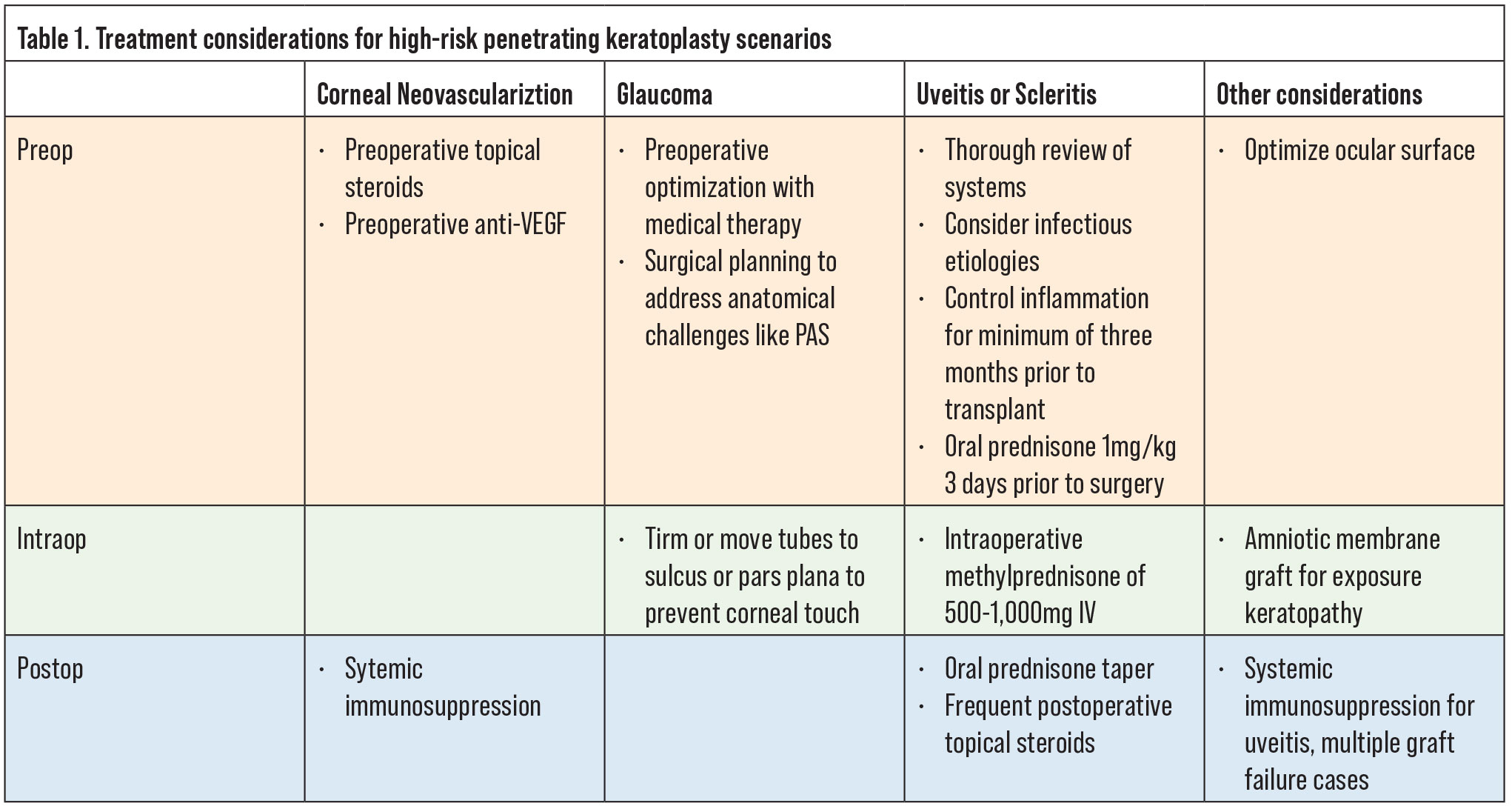
Figure 1. Left: A failed graft related to undiagnosed syphilis. Right: Penetrating keratoplasty for Mooren’s ulcer on mycophenolate mofetil and Humira. |
Additional surgical intervention, such as glaucoma surgery, and postoperative complications can lead to higher graft failure rates. Therefore, as much as is possible, it’s prudent to optimize glaucoma management and other ocular comorbidities prior to keratoplasty to minimize the necessity for subsequent surgical treatment. Careful surgical planning to address anatomical challenges such as peripheral anterior synechiae, an unstable anterior chamber intraocular lens, or a long glaucoma tube near the endothelium either prior to or concurrent with keratoplasty will improve outcomes. Ocular surface conditions such as severe aqueous deficiency syndrome (co-existing with rheumatoid arthritis), floppy eyelids and exposure keratopathy due to lagophthalmos, for example, are regularly overlooked, but must be addressed to ensure epithelial healing. Placing an amniotic membrane graft and temporary tarsorrhaphy can be helpful to promote epithelialization in the crucial immediate postoperative period.
In cases of uveitis or scleritis, the underlying etiology needs to be identified and adequately treated prior to considering surgical intervention. A thorough review of symptoms should be undertaken and a systemic work-up pursued, including evaluating for autoimmune conditions such as rheumatoid arthritis, granulomatosis with polyangiitis, sarcoid, and others as directed by the history and physical exam findings. In addition, infectious etiologies such as tuberculosis and syphilis should be ruled out. If there is latent tuberculosis or syphilis, infectious treatment is critical prior to proceeding with keratoplasty. This approach holds true even for cases of multiple graft failures, as undiagnosed systemic pathology may be the underlying cause of the repeat failures (Figure 1). Once the uveitis has been adequately controlled for at least three months, the patient can undergo transplantation. We generally institute 1 mg/kg of oral prednisone starting three days prior to surgery and for the first week after surgery. In subsequent weeks, we’ll taper the oral prednisone depending on the clinical response of the patient. In addition, we’ll give the patient intraoperative methylprednisolone of 500 mg to 1 gm intravenously. Following the surgery, these patients will also require more frequent and stronger topical steroids (such as difluprednate), to control inflammation.
Because the cornea is an immunologically privileged site, topical steroids are often sufficient for rejection prophylaxis in routine keratoplasty. However, in high-risk transplantation, alternative approaches and adjunctive therapies must be considered (Table 1). HLA matching was previously thought to have a positive effect on graft survival based on retrospective publications.18–20 However, a randomized controlled trial found no evidence of a benefit in HLA matching to prevent immune reactions or graft survival.21 This negative finding was, in part, attributed to a lack of biological relevance of HLA antigens in keratoplasty, specifically, that minor (H) transplantation antigens have stronger associations with graft rejection than the major histocompatibility complex.22,23
Topical tacrolimus has been shown to reduce graft rejection both in rat PKP models and in several human studies.24–26 One retrospective cohort study of high-risk PKP grafts found that those treated with topical tacrolimus 0.03% in addition to topical prednisolone 1% had approximately 50 percent fewer irreversible graft rejections compared with topical prednisolone alone. Another randomized clinical trial of high-risk penetrating keratoplasty patients found a statistically significant reduction in graft rejection to 16 percent among those treated with topical tacrolimus 0.1% compared with 46 percent among those treated with topical cyclosporine 1%.27,29
Antimetabolites may also have some utility in this regard. Specifically, in a prior randomized controlled trial, mycophenolate mofetil (MMF) was relatively well tolerated and showed promise in preventing graft rejection and maintaining graft clarity and survival over a six-month period.28 We’ll often initiate systemic immunosuppression with an antimetabolite such as mycophenolate mofetil or methotrexate, both of which have a relatively good safety profile (Figure 1). These systemic medications take three months to reach therapeutic efficacy so should be initiated preoperatively with enough lead time prior to surgery. Adverse effects should be closely monitored and regular blood tests including liver function tests, creatinine, and CBC with differential performed regularly. Many ophthalmologists work closely with a primary care provider or rheumatologist to initiate these medications and monitor labs.
Therapeutic PK (IK or autoimmune melt)
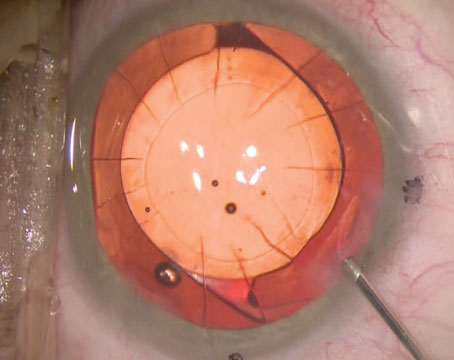
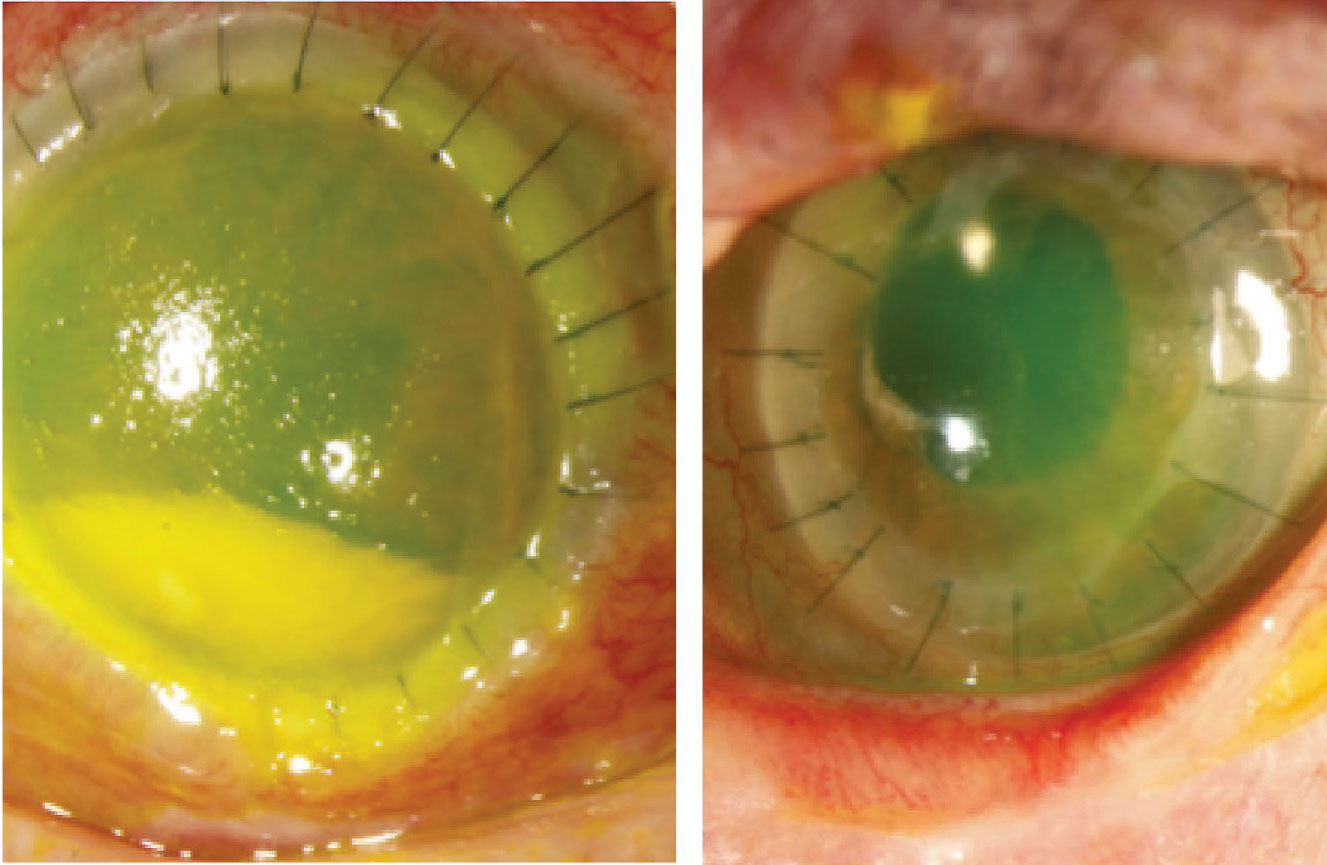
As PKP performed emergently for microbial keratitis has a poor survival prognosis, we favor medical treatment first, resorting to keratoplasty only in severe cases refractory to medical management.29 Despite appropriate anti-microbial therapy, however, corneal perforation or melt may necessitate a therapeutic PKP to preserve globe integrity, and to help eliminate infection (Figure 2).30
These cases can be technically challenging, often requiring irregularly shaped crescentic or large diameter grafts.31,32 A larger trephination into healthy tissue that encompasses the infected tissue improves the chances of clearing the infection while also ensuring wound integrity. Limbus to limbus grafts require a 360-degree conjunctival peritomy which may help preserve corneal limbal stem cells and prevent cornea-scleral sutures from loosening rapidly. A partial thickness trephination at the limbus can allow for a corneoscleral shelf to be created by entering obliquely with a crescent blade. This shelved architecture at the graft-host junction promotes a stable self-sealing wound and preserves the anterior chamber angle. In wounds involving the limbus we favor 9-0 nylon sutures.
Surgical efficiency is important to minimize open globe time and minimize risk of an expulsive hemorrhage or infectious seeding of the anterior chamber. Antimicrobial agents as directed by the underlying infection should be administered, such as intracameral moxifloxacin or voriconazole and subconjunctival antibiotics. Determining when to initiate topical steroids postoperatively is challenging and depends in large part on the underlying infection and the surgeon’s confidence in infection elimination. For bacterial infections, we will often initiate topical steroids right after surgery to prevent worsening inflammation and melt. However, these patients must be monitored closely for signs of recurrent, or worsening, infection.
 |
For PKP in cases of fungal keratitis, determining when and how much topical steroids to use is challenging and we would recommend using them with caution. Topical cyclosporine or tacrolimus may be considered instead, which have immunomodulating mechanisms of action, and also antifungal properties.33 If there is suspicion for fungal spread into the anterior chamber, intracameral voriconazole may be considered. Even with these precautions and close monitoring, PKP in the setting of infectious disease is associated with higher rates of graft failure, and patients should be counseled to maintain realistic expectations, and that a subsequent optical keratoplasty is likely to be required.
In patients presenting for consideration of penetrating keratoplasty due to suspicion of autoimmune melt, a diagnostic work-up to evaluate for underlying conditions (such as rheumatoid arthritis (RA), polyangiitis granulomatosis, relapsing polychondritis, polyarteritis nodosa, etc.) is essential. Surgical intervention without inadequate immunosuppression is likely to significantly worsen the inflammatory response. In these scenarios, systemic therapy with a combination of high dose oral prednisone and steroid-sparing therapy is recommended. It’s important to keep in mind that some of these medications can take time to reach efficacy; mycophenolate can take six to 12 weeks, and methotrexate up to 12 weeks. For these reasons, we often employ preoperative and postoperative courses of high dose oral prednisone, as well as intraoperative solumedrol.
Glaucoma
Glaucoma is both a serious postoperative complication, and a known risk factor for endothelial dysfunction34–37 and graft failure.38 High intraocular pressure not only accelerates the rate of endothelial cell loss, but can also, of course, lead to optic nerve damage and permanent vision loss. Patients with pre-existing glaucoma are known to have two times the rate of graft failure compared with those without.39
Glaucoma medications themselves have been shown to affect endothelial cells differently. Rhopressa, a rho kinase inhibitor, has been found in animal and nonglaucoma studies to enhance endothelial cell wound healing, increase endothelial cell density in animal models, and encourage resolution of corneal edema in patients with Fuchs’ dystrophy.40 Carbonic anhydrase inhibitors, such as dorzolamide, have been suspected to cause endothelial toxicity, though this has yet to be adequately studied.41–43 On the other hand, glaucoma surgeries,44–46 including phacoemulsification, trabeculectomy47,48 and tube-shunt implantation,49,50 have been shown to accelerate endothelial cell loss. Tube-shunt surgery is the most likely to accelerate endothelial cell loss,51 perhaps due to intermittent tube-cornea touch or increased disruption of the blood-aqueous barrier which allows for increased subclinical inflammation.52,53
In patients who had previous glaucoma surgery and have a tube in place, attention should be paid to the length and position of the tube. In cases where the tube is long, and appears to be in close proximity to the endothelium, we recommend trimming it shorter or, in a combined case with our glaucoma team, moving the tube to the sulcus or the pars plana. When possible, we favor pars plana tube repositioning which has been shown to minimize corneal endothelial loss compared to AC location.54
HSV/VZV/CMV Keratitis
Viral keratitis is a common cause of corneal disease, and HSV is one of the leading causes of infectious corneal blindness in developed countries.55 Corneal opacity from viral keratitis is a common indication for PKP; however, recurrence can impact the success of a graft. Herpes simplex virus is ubiquitous in the population, and thought to be latent in corneal tissue,56–58 with rabbit models suggesting potential transmission between host and donor tissue.59 In patients with a known history of HSV keratitis, it’s our practice to start therapeutic doses of oral antivirals, such as 1,000 mg oral Valtrex three times daily or 800 mg oral acyclovir five times daily, starting a week prior to surgery and continuing for at least one month postoperatively. We maintain prophylactic doses of antiviral, such as 1,000 mg Valtrex daily or 800 mg of acyclovir twice daily for life. The Herpetic Eye Disease Study (HEDS) I demonstrated a significant benefit to using oral acyclovir and topical corticosteroids for stromal keratitis.60,61 The HEDS II that followed showed that oral acyclovir decreased the recurrence of HSV keratitis (of any type) by about half.62,63
Some patients are thought to be at increased risk for HSV keratitis after receiving a corneal graft from an HSV positive host.64 Case reports attributing graft failure to HSV are reported in the literature, and it’s likely that HSV infection of endothelial cells can lead to corneal graft failure.65 Cytomegalovirus has also been suspected as a cause of endotheliitis following PKP,66 and donor corneas have been reported as a source of CMV inoculation in previously seronegative recipients.67 Given these possible avenues of infection, particularly in the immediate postoperative period, we recommend vigilance, with a low threshold for starting antiviral therapy at the first signs of possible viral keratitis. For patients with history of prior HSV or varicella zoster virus flares, we recommend initiating a course of antiviral therapy prior to graft implantation, and in the immediate postoperative period.
 |
|
Figure 2. Left: Therapeutic penetrating keratoplasty for Fusarium keratitis resulting in perforation. |
In patients with acute HZO and ocular involvement, more than half have corneal involvement.68 Decreased corneal sensation has been shown to be present in approximately 20 percent of patients presenting with HZO,69 and occurs in nearly half of eyes within the first year.70 With the loss of corneal sensation, the eye experiences tear dysfunction, lower blink frequency, exposure keratopathy and delayed epithelial turnover, making it a strong predictor of poor epithelial healing. For these reasons, we recommend checking corneal sensation in all patients with a history of HZO, and considering amniotic membrane graft and lateral tarsorrhaphy at the time of keratoplasty to promote epithelial healing (Figure 2). If patients have no corneal sensation, keratoplasty should be avoided if possible. We use an identical perioperative oral antiviral regimen as well as a prophylactic oral antiviral for life as described above for HSV. The Zoster Eye Disease Study (ZEDS) is currently underway to determine the effects of suppressive valacyclovir treatment in improving outcomes for patients with herpes zoster ophthalmicus.71
While complex PKPs have higher risks, they also offer the possibility of higher rewards. These transplants are often undertaken in patients with complex ocular disease who are facing severe vision loss and therefore there’s also a high potential to improve vision-related quality of life. Managing patient expectations, ensuring a commitment to close follow up by both provider and patient, as well as a thoughtful approach to perioperative planning can optimize the chance of a satisfying and successful outcome. With these considerations, we have found that, while success in complex PKP isn’t always guaranteed, it is attainable, and can be vision-restoring and life-changing for patients.
Dr. Tran is an ophthalmology resident at the Byers Eye Institute at Stanford.
Dr. Lin is a clinical associate professor of ophthalmology at Stanford.
Dr. Rose-Nussbaumer is an associate professor of ophthalmology at Stanford, and a researcher at the Francis I. Proctor Foundation at UCSF.
1. Thompson RW, Price MO, Bowers PJ, Price FW. Long-term graft survival after penetrating keratoplasty. Ophthalmology 2003;110:7.
2. Sit M, Weisbrod DJ, Naor J, Slomovic AR. Corneal graft outcome study Cornea 2001;20:2.
3. Lim L, Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: Visual outcome and success. Ophthalmology 2000;107:6.
4. Sharif KW, Casey TA. Penetrating keratoplasty for keratoconus: Complications and long-term success. British Journal of Ophthalmology 1991;75:3.
5. Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation 2008;86:12.
6. Fasolo A, Capuzzo C, Fornea M, et al. Risk factors for graft failure after penetrating keratoplasty: 5-year follow-up from the corneal transplant epidemiological study. Cornea 2011;30:12.
7. Eye Bank Association of America. Eye Bank Association of America Statistics Report 2022. Eye Bank Association of America. Published 2022. Accessed June 17, 2023. https://restoresight.org/members/publications/statistical-report/#:~:text=U.S.%20eye%20banks%20reported%20122%2C472,down%203.3%25%20compared%20to%202020.
8. Bersudsky V, Blum-Hareuveni T, Rehany U, Rumelt S. The profile of repeated corneal transplantation. Ophthalmology 2001;108:3:461-469.
9. Matthaei M, Fassin A, Mestanoglu M, et al. Blood-aqueous barrier disruption in penetrating and posterior lamellar keratoplasty: Implications for clinical outcome. Klin Monbl Augenheilkd 2023;240:5:677-682.
10. Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010;117:7:1300-5.e7.
11. Price MO, Thompson RW, Price FW. Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol 2003;121:8:1087-1092.
12. Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology 1994;101:9:1536-1547.
13. Sellami D, Abid S, Bouaouaja G, et al. Epidemiology and risk factors for corneal graft rejection. Transplant Proc 2007;39:8:2609-2611.
14. Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci 2007;48:6:2545-2552.
15. Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci 1998;39:1:18-22.
16. Dratviman-Storobinsky O, Avraham-Lubin BCR, Hasanreisoglu M, Goldenberg-Cohen N. Effect of subconjuctival and intraocular bevacizumab injection on angiogenic gene expression levels in a mouse model of corneal neovascularization. Mol Vis 2009;15:2326-2338.
17. Dohlman TH, McSoley M, Amparo F, et al. Bevacizumab in high-risk corneal transplantation: A pilot multicenter prospective randomized control trial. Ophthalmology 2022;129:8:865-879.
18. Reinhard T, Spelsberg H, Henke L, et al. Long-term results of allogeneic penetrating limbo-keratoplasty in total limbal stem cell deficiency. Ophthalmology 2004;111:4:775-782.
19. Reinhard T, Böhringer D, Enczmann J, et al. Improvement of graft prognosis in penetrating normal-risk keratoplasty by HLA class I and II matching. Eye (Lond) 2004;18:3:269-277.
20. Böhringer D, Daub F, Schwartzkopff J, et al. Operational post-keratopasty graft tolerance due to differential HLAMatchmaker matching. Mol Vis 2010;16:2362-2367.
21. Böhringer D, Grotejohann B, Ihorst G, Reinshagen H, Spierings E, Reinhard T. Rejection prophylaxis in corneal transplant. Dtsch Arztebl Int 2018;115:15:259-265.
22. Nicholls SM, Bradley BB, Easty DL. Effect of mismatches for major histocompatibility complex and minor antigens on corneal graft rejection. Invest Ophthalmol Vis Sci 1991;32:10:2729-2734.
23. Sano Y, Ksander BR, Streilein JW. Minor H, rather than MHC, alloantigens offer the greater barrier to successful orthotopic corneal transplantation in mice. Transpl Immunol 1996;4:1:53-56.
24. Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the management of high-risk corneal and limbal grafts. Ophthalmology 2001;108:10:1838-1844.
25. Ghaffari R, Ghassemi H, Zarei-Ghanavati M, et al. Tacrolimus eye drops as adjunct therapy in severe corneal endothelial rejection refractory to corticosteroids. Cornea 2017;36:10:1195-1199.
26. Zhai LY, Zhang XR, Liu H, Ma Y, Xu HC. Observation of topical tacrolimus on high-risk penetrating keratoplasty patients: A randomized clinical trial study. Eye (Lond) 2020;34:9:1600-1607.
27. Faramarzi A, Abbasi H, Feizi S, et al. Topical 0.03% tacrolimus versus systemic mycophenolate mofetil as adjuncts to systemic corticosteroids for preventing graft rejection after repeat keratoplasty: One-year results of a randomized clinical trial. Eye (Lond) 2021;35:10:2879-2888.
28. Birnbaum F, Mayweg S, Reis A, et al. Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: Long-term results of a prospective, randomised, multicentre study. Eye (Lond) 2009;23:11:2063-2070.
29. Kirkness CM, Ficker LA, Steele AD, Rice NS. The role of penetrating keratoplasty in the management of microbial keratitis. Eye (Lond) 1991;5( Pt 4):425-431.
30. Cristol SM, Alfonso EC, Guildford JH, Roussel TJ, Culbertson WW. Results of large penetrating keratoplasty in microbial keratitis. Cornea 1996;15:6:571-576.
31. Killingsworth DW, Stern GA, Driebe WT, Knapp A, Dragon DM. Results of therapeutic penetrating keratoplasty. Ophthalmology 1993;100:4:534-541.
32. Alfaro Rangel R, Szentmáry N, Lepper S, et al. Large-diameter penetrating keratoplasties are mostly due to very severe infectious keratitis and cannot always prevent secondary enucleation. Klin Monbl Augenheilkd 2022;239:11:1361-1368.
33. Jung JA, Yoon YJ. Development of non-immunosuppressive FK506 Derivatives as antifungal and neurotrophic agents. J Microbiol Biotechnol 2020;30:1:1-10.
34. Kang D, Kaur P, Singh K, Kumar D, Chopra R, Sehgal G. Evaluation and correlation of corneal endothelium parameters with the severity of primary glaucoma. Indian J Ophthalmol 2022;70:10:3540-3543.
35. Melamed S, Ben-Sira I, Ben-Shaul Y. Corneal endothelial changes under induced intraocular pressure elevation: A scanning and transmission electron microscopic study in rabbits. Br J Ophthalmol 1980;64:3:164-169.
36. Gagnon MM, Boisjoly HM, Brunette I, Charest M, Amyot M. Corneal endothelial cell density in glaucoma. Cornea 1997;16:3:314-318.
37. Yu ZY, Wu L, Qu B. Changes in corneal endothelial cell density in patients with primary open-angle glaucoma. World J Clin Cases 2019;7:15:1978-1985.
38. Takemori H, Higashide T, Kobayashi A, Yokogawa H, Sugiyama K. Glaucoma-related risk factors for endothelial cell loss and graft failure after Descemet’s stripping automated endothelial keratoplasty. J Glaucoma. Published online March 30, 2023. doi:10.1097/IJG.0000000000002221
39. Janson BJ, Alward WL, Kwon YH, et al. Glaucoma-associated corneal endothelial cell damage: A review. Surv Ophthalmol 2018;63:4:500-506.
40. Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea 2014;33 Suppl 11:S25-31.
41. Miura K, Ito K, Okawa C, Sugimoto K, Matsunaga K, Uji Y. Comparison of ocular hypotensive effect and safety of brinzolamide and timolol added to latanoprost. J Glaucoma 2008;17:3:233-237.
42. Inoue K, Okugawa K, Oshika T, Amano S. Influence of dorzolamide on corneal endothelium. Jpn J Ophthalmol 2003;47:2:129-133.
43. Kaminski S, Hommer A, Koyuncu D, Biowski R, Barisani T, Baumgartner I. Influence of dorzolamide on corneal thickness, endothelial cell count and corneal sensibility. Acta Ophthalmol Scand 1998;76:1:78-79.
44. Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol 2019;208:211-218.
45. Ianchulev T, Lane S, Masis M, et al. Corneal endothelial cell density and morphology after phacoemulsification in patients with primary open-angle glaucoma and cataracts: 2-Year results of a randomized multicenter trial. Cornea 2019;38:3:325-331.
46. Ko YC, Liu CJ ling, Lau LI, Wu CW, Chou JC, Hsu WM. Factors related to corneal endothelial damage after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg 2008;34:1:46-51.
47. Zarei R, Zarei M, Fakhraie G, et al. Effect of mitomycin-c augmented trabeculectomy on corneal endothelial cells. J Ophthalmic Vis Res 2015;10:3:257-262.
48. Smith DL, Skuta GL, Lindenmuth KA, Musch DC, Bergstrom TJ. The effect of glaucoma filtering surgery on corneal endothelial cell density. Ophthalmic Surg 1991;22:5:251-255.
49. Lee EK, Yun YJ, Lee JE, Yim JH, Kim CS. Changes in corneal endothelial cells after Ahmed glaucoma valve implantation: 2-year follow-up. Am J Ophthalmol 2009;148:3:361-367.
50. Kim KN, Lee SB, Lee YH, Lee JJ, Lim H Bin, Kim CS. Changes in corneal endothelial cell density and the cumulative risk of corneal decompensation after Ahmed glaucoma valve implantation. Br J Ophthalmol 2016;100:7:933-938.
51. Kim MS, Kim KN, Kim CS. Changes in corneal endothelial cell after Ahmed Glaucoma valve implantation and trabeculectomy: 1-Year Follow-up. Korean J Ophthalmol 2016;30:6:416-425.
52. Banitt MR, Sidoti PA, Gentile RC, et al. Pars plana Baerveldt implantation for refractory childhood glaucomas. J Glaucoma 2009;18:5:412-417.
53. Hau S, Barton K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol 2009;20:2:131-136.
54. Tojo N, Hayashi A, Consolvo-Ueda T, Yanagisawa S. Baerveldt surgery outcomes: Anterior chamber insertion versus vitreous cavity insertion. Graefes Arch Clin Exp Ophthalmol 2018;256:11:2191-2200.
55. Liesegang TJ, Melton LJ, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol 1989;107:8:1155-1159.
56. Cook SD, Ophth FC, Hill JH. Herpes simplex virus: Molecular biology and the possibility of corneal latency. Surv Ophthalmol 1991;36:2.
57. O’Brien WJ, Tsao LS, Taylor JL. Tissue-specific accumulation of latency-associated transcripts in herpes virus-infected rabbits. Invest Ophthalmol Vis Sci 1998;39:10:1847-1853.
58. Kaye SB, Lynas C, Patterson A, Risk JM, McCarthy K, Hart CA. Evidence for herpes simplex viral latency in the human cornea. Br J Ophthalmol 1991;75:4:195-200.
59. Zheng X. Reactivation and donor-host transmission of herpes simplex virus after corneal transplantation. Cornea 2002;21(7 Suppl):S90-3.
60. Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study: A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology 2020;127:4S:S5-S18.
61. Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 1994;101:12:1871-1882.
62. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med 1998;339:5:300-306.
63. Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: The effect of oral antiviral prophylaxis. Arch Ophthalmol 2010;128:9:1178-1183.
64. Remeijer L, Doornenbal P, Geerards AJ, Rijneveld WA, Beekhuis WH. Newly acquired herpes simplex virus keratitis after penetrating keratoplasty. Ophthalmology 1997;104:4:648-652.
65. De Kesel RJ, Koppen C, Ieven M, Zeyen T. Primary graft failure caused by herpes simplex virus type 1. Cornea 2001;20:2:187-190.
66. Sonoyama H, Araki-Sasaki K, Osakabe Y, et al. Detection of cytomegalovirus DNA from cytomegalovirus corneal endotheliitis after penetrating keratoplasty. Cornea 2010;29:6:683-685.
67. Holland EJ, Bennett SR, Brannian R, Osborne JC, Goeken JA, Krachmer JH. The risk of cytomegalovirus transmission by penetrating keratoplasty. Am J Ophthalmol 1988;105:4:357-360.
68. Niederer RL, Meyer JJ, Liu K, Danesh-Meyer H V. Herpes zoster ophthalmicus clinical presentation and risk factors for loss of vision. Am J Ophthalmol 2021;226:83-89.
69. Cobo M, Foulks GN, Liesegang T, et al. Observations on the natural history of herpes zoster ophthalmicus. Curr Eye Res 1987;6:1:195-199.
70. Cobo LM. Corneal complications of herpes zoster ophthalmicus. Prevention and treatment. Cornea 1988;7:1:50-56.
71. Cohen EJ, Hochman JS, Troxel AB, Colby KA, Jeng BH, ZEDS Trial Research Group. Zoster Eye Disease Study: Rationale and Design. Cornea 2022;41:5:562-571.