Only 10 in 100,000 adults and three in 100,000 children are diagnosed with noninfectious posterior uveitis.1 But interest in this often intractable, sight-threatening condition has never been greater. With FDA approval of two new therapeutic agents—the biologic adalimumab (Humira, AbbVie) in June of 2016 and a 0.18-mg fluocinolone acetonide intravitreal insert (Yutiq, EyePoint Pharmaceuticals) in October of 2018—specialists and general ophthalmologists are evaluating new data and continuing to explore best approaches to treatment.
In this report, experts offer guidance on advances in therapies, complications and optimized inflammation control, which is critical to saving vision and improving quality of life.2 Cost, insurance coverage, efficacy and patient preferences need to be considered for this critical condition, which accounts for 5 percent to 20 percent of legal blindness.3
Initial Presentation
Recall that noninfectious posterior uveitis, associated with complex comorbidities, typically results from a T-cell-mediated autoimmune process that involves proinflammatory cytokines.4 The condition encompasses diverse and often difficult-to-control inflammatory disorders, including spondyloarthropathies, Behçet’s disease, sarcoidosis, juvenile chronic arthritis, Vogt-Koyanagi-Harada syndrome, immune recovery syndrome, uveitis with tubulointerstitial disease and others.5 When you see a patient with suspected posterior uveitis, experts urge you to combine a complete medical and social history with a comprehensive dilated eye examination and, as needed, ancillary studies, including optical coherence tomography and fluorescein angiography, to help confirm and classify the primary site of inflammation. Conduct laboratory workups of possible systemic inflammatory, infectious and malignant etiologies.
Akshay Thomas, MD, MS, a retina and uveitis specialist at Tennessee Retina, also applies the following approach to therapy. His advice:
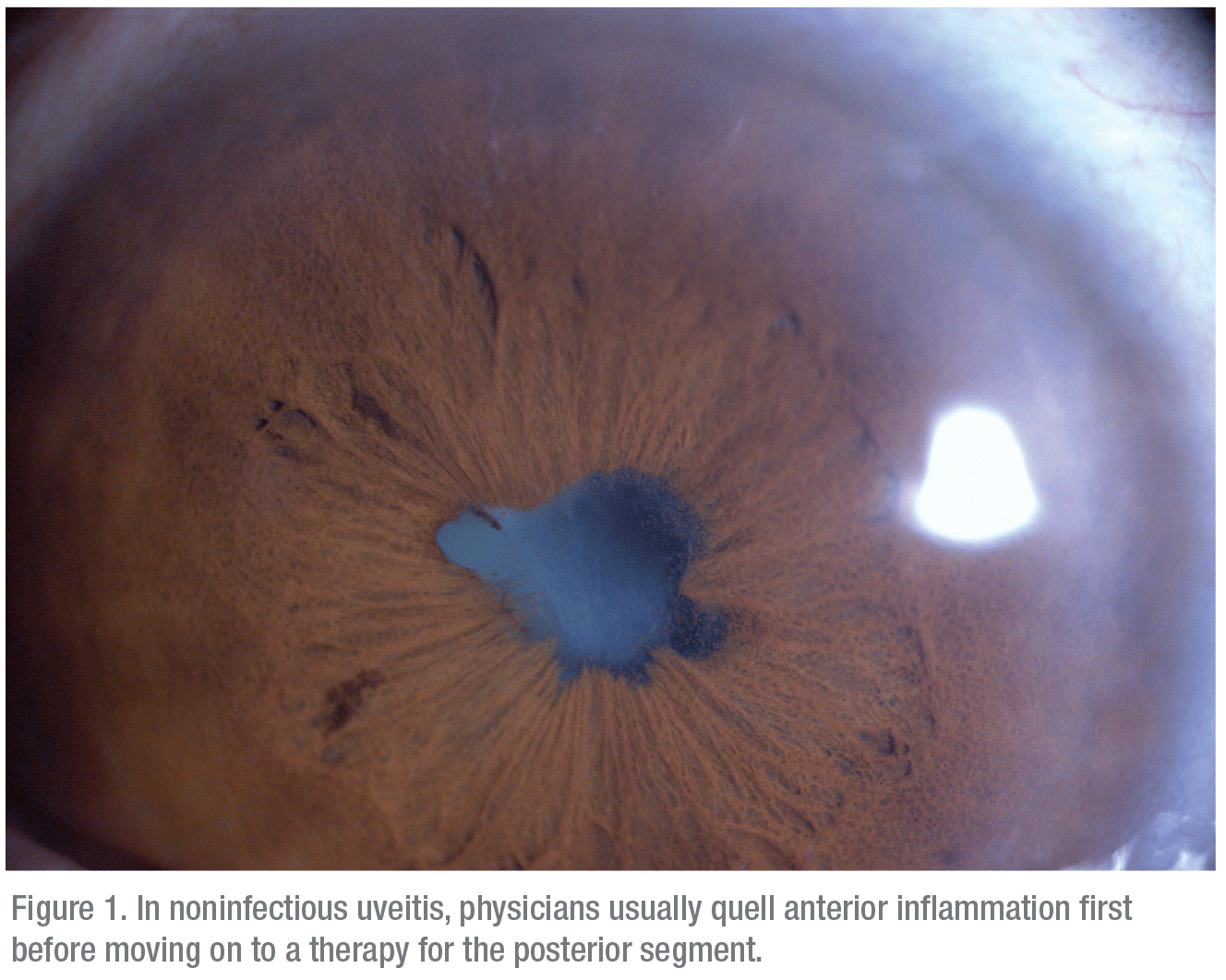 |
1. Rule out any infectious cause.
2. Rule out syphilis, via laboratory evaluation.
3. Test for tuberculosis and Lyme disease in patients with risk factors for exposure.
4. Rule out a herpetic cause or toxoplasma in patients with retinitis.
5. Withhold heavy oral corticosteroids—certainly injection—until you’ve ruled out an infectious etiology of the inflammation.
6. Consider topical steroids, pending laboratory results.
If your patient has infectious uveitis, appropriate antimicrobial, antiviral or antiparasitic therapy needs to be initiated. For infectious retinitis, oral steroids may be added once the retinitis appears to be consolidating. (Dr. Thomas says he almost never uses local steroid injections in a patient with infectious uveitis.)
After Infection’s Ruled Out
After confirming a diagnosis of noninfectious posterior uveitis, Dr. Thomas says that the degree of inflammation informs treatment.
“If the predominant sites of inflammation are in the anterior chamber—with or without cystoid macular edema—use of topical steroids, either prednisolone acetate or difluprednate acetate emulsion (Durezol), may be appropriate initially,” he notes.6 “For significant vitritis, retinal vasculitis, significant CME, choroiditis or chorioretinitis, topical therapy alone may be insufficient. In such eyes with either unilateral disease or very asymmetric disease, local steroid injections, implants and inserts can be quite effective.7 This therapy provides a rapid response and avoids the side effects of systemic medications. In eyes with significant bilateral disease, I usually enlist systemic therapy, if the patient can tolerate it. This typically involves a course of oral steroids with a taper; if inflammation flares during the taper, the patient may need to be started on immunomodulatory therapy. The only such agent FDA-approved for non-infectious uveitis is Humira. However, many other systemic agents can be employed.”
If a patient with chronic bilateral disease can’t tolerate systemic therapy, a long-acting local steroid implant (0.59 mg fluocinolone acetonide implant, Retisert, Bausch + Lomb) or insert (Yutiq) may be needed.
“If you have a uveitis patient whom you treat episodically, only when he or she flares, you need to know when to switch to long-term inflammatory control,” he says. “With each flare, the patient’s visual potential may decline. If your approach to a chronic recurrent panuveitis is reactive—only treating during flares—your patient may experience a saw-tooth pattern of visual decline, including progressive retinal/optic atrophy, a compromised angle with secondary glaucoma or ultimately hypotony from fibrosis along the ciliary processes.”
Finding Your Way
The initial treatment path isn’t always clear. “Determining how traditional local treatments should fit into your armamentarium is a challenge because comparative trial data don’t exist,” says uveitis specialist Thomas A. Albini, MD, a professor of clinical ophthalmology who specializes in medical retina and vitreoretinal surgery at Bascom Palmer Eye Institute at the University of Miami. “We don’t have head-to-head comparisons of these treatments. Choosing the right therapy often depends on anecdotal experience—and the related experiences from other practitioners.”
Corticosteroid drops provide limited efficacy in the posterior segment. “Some data support using Durezol, which can provide limited efficacy, helping control CME. Otherwise, topicals have limited effect deeper than anterior uveitis.”6
Dr. Albini notes that, in the recent POINT study,7 Ozurdex and intravitreal triamcinolone demonstrated a greater and faster therapeutic effect on uveitic macular edema than periocular Kenalog. No significant differences between Ozurdex and intravitreal triamcinolone were found.
Despite these findings, Sam Dahr, MD, clinical professor at the Dean McGee Eye Institute and University of Oklahoma College of Medicine in Oklahoma City, utilizes both Ozurdex and intravitreal triamcinolone. “Intravitreal steroids can rapidly quiet a ‘hot eye’ and buy time for steroid-sparing therapy to take effect,” he says. “It’s often effective for anatomic and angiographic macular edema, as well as angiographic vasculitis. If a prospective cataract surgery patient with uveitis can’t take perioperative oral prednisone, intravitreal steroids one to two weeks before surgery is an effective perioperative regimen.”
When monitoring patients who have received the Ozurdex implant, Dr. Dahr finds IOP predictably peaks “in the mid 20s” at 45 to 60 days. “I often see a cumulative and additive IOP effect after repeated sub-Tenon’s injections or intravitreal triamcinolone, but less often when using sequential Ozurdex,” he says. “I try to minimize sequential local steroid injections by utilizing steroid-sparing therapy, but sometimes I give a patient three to four injections in the first 12 to 18 months of fine-tuning a steroid-sparing regimen.”
Dr. Albini acknowledges that Ozurdex may have a better side-effect profile than triamcinolone acetonide because of a gentler effect on IOP. It also lasts up to six months, compared to triamcinolone acetonide’s three-month duration of action.
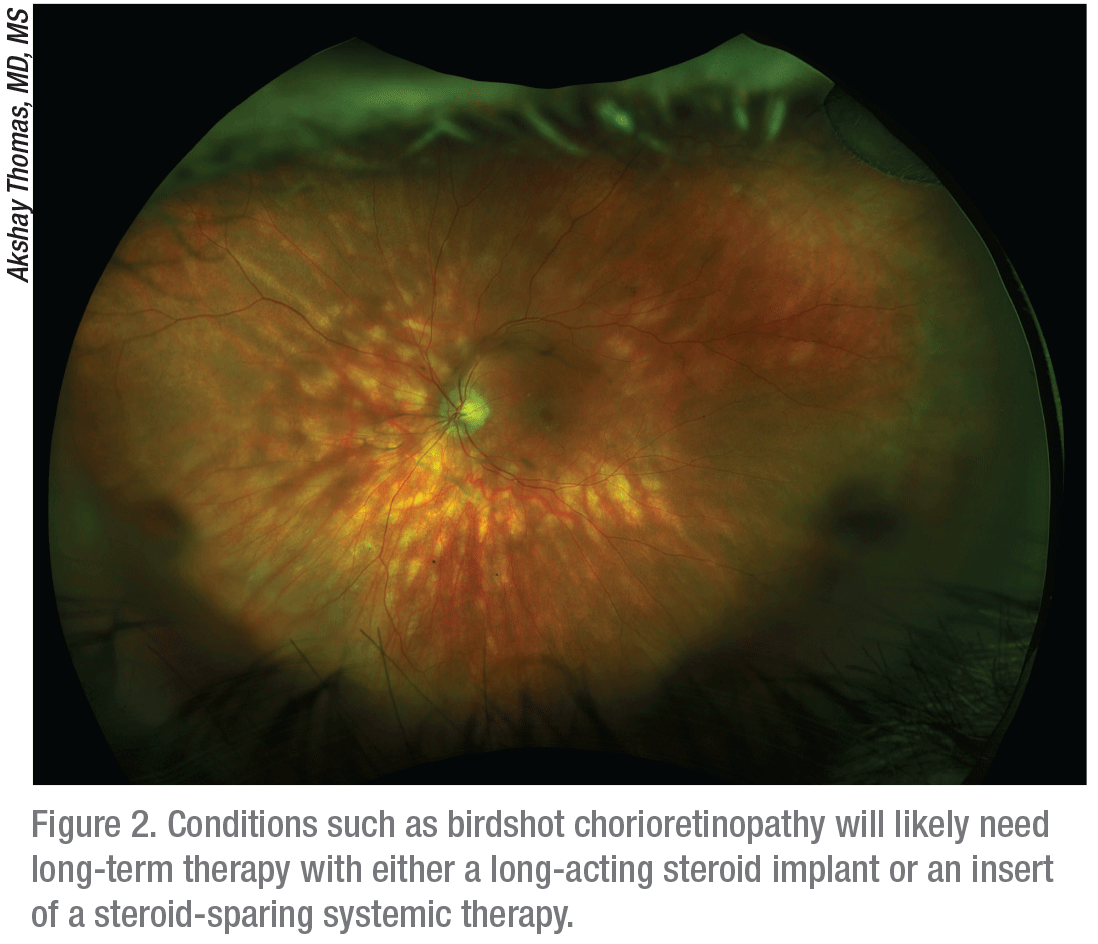 |
“The dexamethasone in Ozurdex doesn’t disperse and block vision, or spread into the anterior segment and cause a pseudo-hypopyon, blocking the trabecular meshwork, as triamcinolone does, increasing IOP,” he explains. Despite Ozurdex’s advantages, however, Dr. Albini remains mindful of the POINT study results, which showed a small statistically insignificant difference in outcome when comparing Ozurdex to two consecutive triamcinolone acetonide treatments. “It’s difficult to decide between these treatments,” he admits. “Ozurdex has subtle advantages, but it’s also eight times more expensive than intravitreal triamcinolone, an important consideration if Ozurdex is not fully reimbursed by insurance. Also, we employ intravitreals for more difficult posterior disease involving macular edema or vascular leakage. Intravitreals are much better than systemic medications in these cases, in my view. The problem with the intravitreals is their limited course and ocular side effects, such as glaucoma and cataracts.”
Although the POINT study found intravitreal treatments reduced uveitis macular edema more effectively than periocular Kenalog, Dr. Albini says he continues to rely on Kenalog as well. “I don’t believe there is clear evidence that periocular Kenalog should be removed from our armamentarium, or that the intravitreal injections always provide greater ef fects. However, I do think we need to consider the risk of endophthalmitis or patient discomfort associated with an Ozurdex implant injection. In general, however, treatment choices also vary from patient to patient and among physicians. A lot of what we do is based on personal experiences and preferences.”
Sustained-release Fluocinolone
Retisert, the long-acting intravitreal implant, and the recently approved Yutiq provide compelling alternative treatments for appropriate patients. Here’s a look at their benefits and risks.
• Retisert. “The two-year data from the Multicenter Uveitis Steroid Treatment (MUST) trial favored the Retisert implant over traditional systemic immunomodulatory therapy for the management of non-infectious intermediate, posterior and panuveitis,” says Dr. Thomas.8 “The five-year MUST data showed similar outcomes between the groups. However, the seven-year MUST data favored systemic, steroid-sparing therapy. Remember that the MUST study was only designed to be a two-year study and that the five-year and seven-year data were only observational. A Retisert implant is only expected to provide inflammatory control for up to three years, after which a Retisert exchange may be needed.”9
Dr. Albini says a fluocinolone acetonide implant is very effective for bad cases “if you want to avoid immunosuppressant therapy, such as for a pregnant patient, who would not be a candidate for immunosuppression therapy.” He adds: “Retisert also seems to be a very viable alternative to systemic therapy. It works well over the life of the implant.”
In terms of uveitis recurrences, in the clinical trial data submitted for FDA approval, in two randomized, double-masked, multicenter controlled clinical trials, 227 patients with chronic non-infectious uveitis (a one-year or greater history) affecting the posterior segment of one or both eyes received a 0.59-mg Retisert. In terms of the primary endpoint, recurrence, the rates of recurrence ranged from approximately 7 percent (7/108) to 14 percent (16/116) for the 34-week post-implantation period as compared to approximately 40 percent (46/116) to 54 percent (58/108) for the 34-week pre-implantation period.10
According to the labeling, within 34 weeks post-implantation, approximately 60 percent of patients will require IOP-lowering medications to control intraocular pressure. Within an average post-implantation period of about two years, approximately 32 percent of patients are expected to require filtering procedures to control intraocular pressure. Also, within approximately two years post-implantation, “nearly all phakic eyes are expected to develop cataracts and require cataract surgery.”10
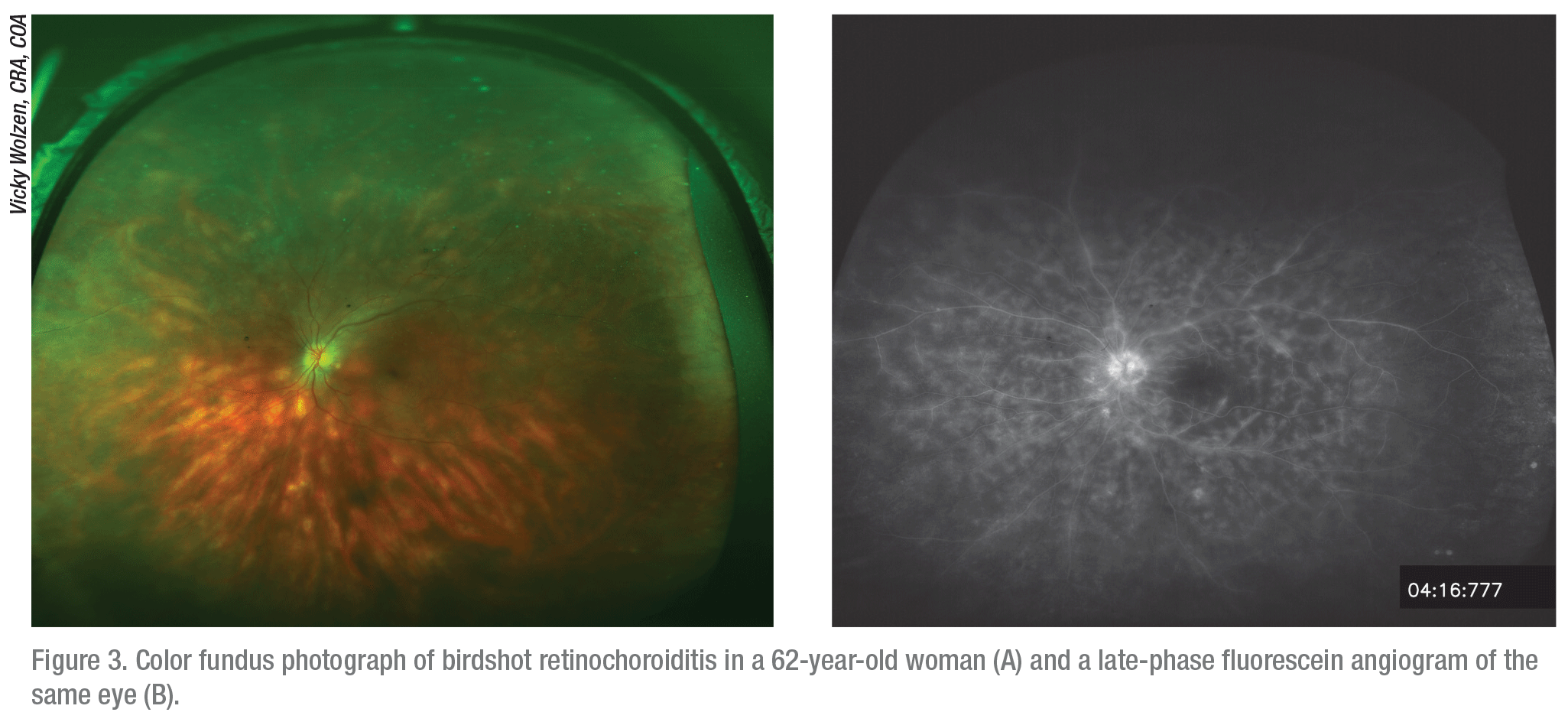 |
When considering the Retisert implant, Dr. Thomas determines if the patient has demonstrated a good response to local steroids. “I also make sure the patient understands the effects of long-term local steroid therapy,” he continues. “The MUST data showed that essentially all patients with a Retisert developed a cataract, up to 77 percent of patients needed IOP-lowering drops and 37 percent of patients required filtering surgery within three years of Retisert implantation.”11
Dr. Thomas notes that surgically implanting Retisert is a simple procedure. “Exchanging the old implant for a new one every three years can be more challenging and may need to be done by a vitreoretinal specialist,” he adds.12,13 “Some physicians may put a second implant into the eye without replacing the old one. This might not be an ideal practice as it uses up valuable real estate and the older Retiserts may be at risk of dissociation or dislocation.”12
In his practice, Dr. Albini has a straightforward message for patients who are considering Retisert: “You are buying three surgeries (surgical implantation and possible glaucoma and cataract procedures),” he says. “If you’re lucky, maybe two surgeries. But you’re saving yourself from being on a systemic medication. Most patients do well with systemic immnosuppression, but some avoid it because of possible side effects, such as nausea, fatigue, bone marrow suppressions or liver changes.”
For most of these patients, Dr. Albini says a second implant is put in place when the inflammation of their uveitis recurs five years after placement of the first implant. “Waiting for recurrence of inflammation might not be the best approach,” he says. “It might be best to put in another implant after the implant’s three-year release of the steroid. But that involves surgery and more accompanying risks.”
• Yutiq. The FDA approved the Yutiq insert after a multicenter, randomized, prospective, double-masked, sham-controlled, three-year Phase III clinical trial found the insert’s uveitis recurrence rates at 36 months to be 56.3 percent for Yutiq, which was significantly lower than that of sham-treated eyes (92.9 percent).14 Intraocular pressure-lowering drops were used in 42 percent of Yutiq-treated eyes and a third of sham-treated eyes. IOP-lowering surgery was performed in 6 percent of Yutiq-treated eyes and 12 percent of sham-treated eyes. Seventy-four percent of Yutiq patients required cataract surgery vs. 24 percent of sham patients.14
“In one study, 12-month data showed a reduction in inflammation and uveitis flares in eyes randomized to receive the insert over sham,” says Dr. Thomas.15 “Of note, the rate of use of IOP-lowering medications over the study was similar between the groups. However, how this compares to steroid-sparing systemic therapy or the Retisert implant remains to be seen.”
Yutiq is injected in the clinic, allowing it to float freely in the cavity, instead of being sutured into the eye. Unlike the dissolvable dexamethasone implant Ozurdex, however,
Yutiq is packaged with polyethylene and therefore isn’t degradable.
“After a patient potentially undergoes Yutiq injections every three years during a 10-year span, the question will be: How and when are we going to remove the leftover inserts from the eye?” asks Dr. Albini. “This is an issue that has to be addressed.”
Yutiq became available in March of 2019. Dr. Dahr is performing initial Yutiq injections in patients who need continued intravitreal steroid therapy despite his best efforts at utilizing steroid-sparing therapy.
 |
Often, Dr. Dahr says, noninfectious posterior uveitis patients have recurrent macular edema, despite steroid-sparing therapy and a clinically controlled eye. Those patients’ edema will often respond to an intravitreal steroid, he says. “Yutiq is an option for patients who can’t tolerate or who are ineligible for steroid-sparing therapy,” he says. “I may also eventually use Yutiq when a patient has improved but is not fully controlled on traditional steroid-sparing treatment. For tough patients who can’t be fully controlled on monotherapy with a traditional agent, choices for combination therapy may include the traditional agent plus Yutiq or the traditional agent plus Humira. Here, a discussion with the patient regarding individual preferences is critical.”
Sustained Immunosuppression
Dr. Albini points out that many patients with noninfectious posterior uveitis may have chronic diseases, such as birdshot chorioretinopathy, Behçet’s syndrome, sympathetic ophthalmia, Vogt-Koyanagi-Harada syndrome, serpiginous choroidopathy and multifocal choroiditis with panuveitis.
“A more chronic course is indicated in these cases,” Dr. Albini says. “If these conditions aren’t treated properly, the affected patients will most often lose their vision.”
 |
For this reason, he and other specialists urge providers to involve specialists in these cases. “Sustained immunosuppression is what is needed,” Dr. Albini says.16 “A retina specialist or uveitis specialist will need to provide this type of chronic care. Sustained immunosuppression is beyond a general ophthalmologist’s typical purview. Most general ophthalmologists would only treat anterior uveitis.” (See “Role of the General Ophthalmologist in Chronic Care” on page 26.”)
Dr. Dahr warns against waiting too long to initiate a patient on some form of steroid-sparing therapy. “The key in these cases is to commit patients with significant disease to steroid-sparing therapy early in their course rather than to wait one to two years, when a breakdown of the blood-ocular barrier makes achieving long-term control and possible remission much harder.”
Maximizing Your Options
In June 2016, Humira, an inhibitor of tumor necrosis factor-alpha (TNFα), became the only systemic non-corticosteroid agent approved by the FDA for the treatment of non-infectious uveitis.17 Infliximab is the other agent from this drug class that has been used effectively to quiet the symptoms of Behçet’s syndrome, which, as mentioned previously, is one of many inflammatory conditions associated with posterior noninfectious uveitis.18
Several off-label immunosuppressive antimetabolites have also become important in the treatment of chronic or recurrent noninfectious posterior uveitis–and will continue to play a role, experts say. These agents include methotrexate, mycophenolate mofetil (CellCept) and azathioprine (Imuran). “Specialists have been treating with methotrexate and CellCept for a decade or more,” says Dr. Albini. “At this point, it’s not definitively clear which one is better.19 More comparative trial data is coming in the literature.” Other off-label steroid-sparing treatments include such T-cell inhibitors as cyclosporine and tacrolimus (Prograf).
Antimetabolites With Humira
For Dr. Dahr, antimetabolite therapy remains a mainstay that has only been enhanced by the recent availability of Humira. 20 “Antimetabolite monotherapy will achieve long-term control in approximately one-third of cases,” he says. “Now, the combination of an antimetabolite (methotrexate, mycophenolate or azathioprine) with Humira has become very popular. Antimetabolites have 30 years of literature supporting their use for uveitis. Community rheumatologists are very comfortable with the methotrexate/Humira combination, and they can help the ophthalmologist.”
According to Dr. Dahr, in addition to its intrinsic immunomodulatory effect, methotrexate may enhance the effect of Humira by slowing the clearance of Humira and reducing the development of neutralizing antibodies to Humira. “Some patients read the Humira label and wish to avoid the drug but are willing and able to take the antimetabolite, so the antimetabolite can be used with local steroid options,” says Dr. Dahr. “Some patients may not tolerate antimetabolites but do well with Humira monotherapy, or Humira monotherapy plus an intravitreal steroid. The physician and patient must discuss combination approaches.”
Managing Costs
When considering monotherapy in the clinic, Dr. Albini says a potentially complicated choice sometimes needs to be made between Humira and a traditional immunosuppressant such as methotrexate. “When making this choice, we need to consider that methotrexate costs $50 month and Humira costs about $6,000 a month,” says Dr. Albini. “Payors have different policies on reimbursing for Humira, so be aware of the policies that are being followed by a particular third-party payor. Some companies will pay for Humira only when the patient is at risk of liver disease or other conditions that would contraindicate treatment with methotrexate. Some payors will pay for Humira if your patient experiences a documented failure from another treatment. Some payors won’t pay at all. Others will pay as soon as they receive the diagnosis.”
Favoring First-line Treatments
Dr. Albini says most uveitis specialists are more comfortable with traditional immunosuppression as first-line therapy. “This is significant because Humira was a second-line or third-line treatment before it was approved for uveitis,” he points out. “Now that it’s approved, it’s a first-line treatment.” He now decides which treatments to choose based on the safety data of long-term use associated with the traditional agents compared to the limited amount of data associated with Humira. “Cost also is obviously a big factor to consider for many patients,” he adds.
However, some patients only want to receive the FDA-approved medication. “Some also want to avoid methotrexate’s required blood monitoring four to six times per year,” says Dr.
Albini. “As a result, traditional treatments have often become more difficult to provide than Humira.”
He also notes which patients have liver toxicity or are trying to get pregnant. “I use Humira for these patients,” he says. “I prefer Humira for a woman of child-bearing age. Methotrexate and CellCept are totally contraindicated for women who are or plan to get pregnant.”
T-Cell Inhibitors + Cyclosporine
Dr. Dahr says the combination of an antimetabolite and T-cell inhibitor such as cyclosporine or tacrolimus (Prograf) also remains an option.
“I see quite a bit of new-onset VKH disease in patients in their 20s,” he explains. “I often put them on an antimetabolite, plus the T-cell inhibitor at the initial presentation. I then taper their oral steroid to 10 mg within three months and taper the last 10 mg of steroid over 12 months or so. If the eyes are quiet, I may then taper the steroid-sparing therapy over the subsequent 12 to 24 months.” Dr. Dahr has found this “three-year plan” lets him avoid the local corticosteroids for VKH patients, who he says seem prone to corticosteroid-related IOP issues. Finally, the future may see a role for the interleukin-6 (IL-6) receptor inhibitor tocilizumab (Actemra), used to treat moderate to severe rheumatoid arthritis in children and adults. “Tocilizumab has shown encouraging initial data21 but doesn’t carry an FDA label for uveitis,” Dr. Dahr notes. “It will be difficult to procure in a community setting because of reimbursement issues.”
Continuing Progress
The future care of patients with this potentially blinding condition looks brighter, as researchers and clinicians continue to progress in the therapeutic arena.
“We now have a very good armamentarium that we can use to help these patients,” says Dr. Dahr. “However, it’s important to realize there will never be a ‘magic bullet’ drug for uveitis—or for autoimmune disease, in general. Still,the vast majority of patients can be controlled with our current therapies, albeit often in combination.” REVIEW
Neither Dr. Dhar nor Dr. Thomas have declared a financial interest related to this article. Dr. Albini has consulted for Bausch + Lomb.
1. Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: A claims-based analysis. JAMA Ophthalmol 2016;134:1237-1245.
2. Brady CJ, Villanti AC, Law HA, et al. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst Rev. 2016:12;2.
3. Frick KD, Drye LT, Kempen JH, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci 2012;53:3:1169-76.
4. de Smet MD, Taylor SR, Bodaghi B, et al. Understanding uveitis: The impact of research on visual outcomes. Prog Retin Eye Res 2011;30:6:452-470.
5. Pan J, Kapur M, McCallum R. Noninfectious immune-mediated uveitis and ocular inflammation. Curr Allergy Asthma Rep.2014;14:1:409.
6. Mulki L, Foster CS. Difluprednate for inflammatory eye disorders. Drugs Today (Barc). 2011;47:5::327-33.
7. Thorne JE, Sugar EA, olbrook JT, et al. Periocular triamcinolone vs. intravitreal triamcinolone vs. intravitreal dexamethasone implant for the Tteatment of uveitic macular edema: the PeriOcular vs. INTravitreal corticosteroids for uveitic macular edema (POINT) Trial. Ophthalmology 2019;126:2:283-295.
8. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, et al. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: Ffty-four-month results of the multicenter uveitis steroid treatment (MUST) trial and follow-Up study. Ophthalmology 2015;122:10:1967-75
9. Kempen JH, Altaweel MM, Holbrook JT, et al. Association between long-lasting intravitreous fluocinolone acetonide implant versus systemic anti-inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA 2017;317:1993-2005.
10. etisert abeling. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021737s007lbl.pdf. Accessed 16 July 2019.
11. Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: Three-year clinical trial results. Arch Ophthalmol 2008;126:9:1191-201.
12. Holbrook JT, Sugar EA, Burke AE , et al. Dissociations of the fluocinolone acetonide implant: The multicenter uveitis steroid treatment (MUST) trial and follow-up study. Am J Ophthalmol 2016;164:29-36.
13. Wang H, Hardin J, Kaintatzis A, et al. Inadvertent expulsion of fluocinolone acetonide intravitreal implant during pars plana vitrectomy. Ophthalmol Retina 2018;2:1:75-77.
14. Yutiq Phase III data announcement, May 1, 2019. EyePoint Pharmaceuticals.
15. Jaffe GJ, Foster CS, Pavesio CE, et al. Effect of an injectable fluocinolone acetonide insert on recurrence rates in chronic noninfectious Uveitis affecting the posterior segment: Twelve-month results. Ophthalmology 2019;126:4:601-610.
16. Jabs DA. Immunosuppression for the uveitides. Ophthalmology 2018;125:2:193-202.
17. LaMattina KC, Goldstein DA. Adalimumab for the treatment of uveitis. Expert Rev Clin Immunol 2017;13:3:181-188.
18. Thomas AS. Biologics for the treatment of noninfectious uveitis: Current concepts and emerging therapeutics. Curr Opin Ophthalmol 2019;30:3:138-150.
19. Rathinam SR, Babu M, Thundikandy R. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology 2014;121:10:1863-70
20. Ramanan AV, Dick AD, Benton D. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial). Trials 2014 9;15:14.
21. Sepah YJ, Sadiq MA, Chu DS, et al. Primary (Month-6) outcomes of the stop-uveitis study: Evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol. 2017;183:71-80.



