Here, I'd like to provide an update on what we've been learning and offer some suggestions for improving the accuracy of IOP measurements.
The Biomechanics Factor
The most eye-opening development in the past few years has been the revelation that corneal biomechanics—the physical characteristics of corneal tissue—may affect the accuracy of intraocular pressure applanation measurement as much as, or more than, CCT. Much of the key work in this area has been done by Cynthia Roberts, PhD, a biomedical engineer at Ohio State University.1
Goldmann applanation tonometry measures IOP by flattening the cornea, which is not neutral in this measurement. Dr. Roberts has shown that factors affecting corneal resistance include structural considerations, such as the amount of rigidity produced by the way the collagen beams in the tissue line up. The "bendability" of corneal tissue can also be affected by short-term factors such as the presence of corneal edema.
As a result of this new perspective, researchers are studying hysteresis, one measure of corneal biomechanical properties. Currently, the only instrument that can measure hysteresis is the Reichert Ocular Response Analyzer, which also measures corneal resistance, CCT and traditional (Goldmann) IOP, and provides a "compensated" IOP, less affected by corneal properties. (Our practice recently purchased a unit, and we're hoping to successfully incorporate its measurements into our IOP calculations.) Another way of gauging the cornea's biomechanical properties, currently being looked at by researchers, is Young's Modulus. This is a measure of stiffness that helps to predict how a material under pressure will behave.
Of course, another approach to this problem is to find a way to measure IOP that is unaffected by the cornea's thickness or biomechanics. The Pascal Dynamic Contour Tonometer (Zeimer Ophthalmics) is a digital tonometer that uses the principle of contour matching instead of applanation to measure IOP. A recent prospective, in vivo clinical study reported at the 2006 Association for Research in Vision and Ophthalmology meeting found that IOP measured by the DCT was within 1 mmHg of the intracameral pressure measured manometrically with a reference pressure sensor, over a wide range of pressures. (A. Weber et al; 2006 ARVO E-abstract 4444.)
The Pascal DCT could be the future gold standard for measuring IOP. However, even if this turns out to be the case, it will take a number of years for the DCT to replace Goldmann tonometry, for several reasons. First, we're all accustomed to using the Goldmann instrument. Second, the DCT costs more than $5,000 per unit, so making the switch requires a substantial investment, especially if you plan to place it in multiple lanes. Third, because the DCT readings may differ from the Goldmann readings, it will require switching to a new set of standards; IOP numbers will mean something different from what we're accustomed to. So for now, clinicians are likely to continue using Goldmann and adjusting the numbers on the basis of CCT.
Making the Most of Goldmann
Until an alternative to Goldmann applanation tonometry becomes popular, here are a few strategies to help ensure that you get the best outcomes using this familiar technology.
• Don't rely too much on conversion tables. In the past few years, many MDs have come to rely on conversion tables that use an algorithm to adjust IOP measured with Goldmann, based on CCT. What we've learned about corneal biomechanics indicates that this approach isn't necessarily reliable. It may produce an accurate IOP for many patients—but there's no way to know which patients those are. One 580-µm cornea may be altering the IOP reading very differently than another does.
• Make the patient blink right up until you measure CCT. If you anesthetize the eye, patients tend to lose their blink response, and it only takes 15 to 30 seconds for a cornea to begin drying out, causing it to become thinner.
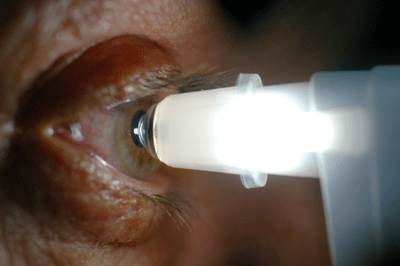 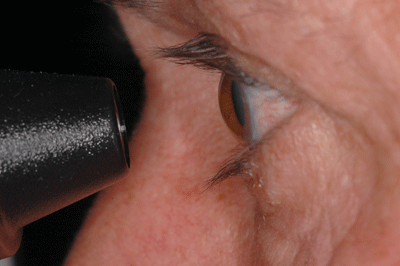 |
| The Dynamic Contour Tonometer from Zeimer Ophthalmics (left) uses contour matching instead of applanation to measure IOP, minimizing the impact of corneal characteristics on the measurement. Reichert's Ocular Response Analyzer (right) measures the cornea's biomechanical properties, which may make it possible to compensate for their effect on IOP measurement. |
• Measure CCT before performing gonioscopy. The contact lens you place on the eye to perform gonioscopy will slightly alter the cornea. So do gonioscopy after you've taken your corneal measurements.
• Think of CCT as falling into three categories. While corneal biomechanics may make it difficult to be certain of the accuracy of Goldmann measurements, simply noting that a cornea is especially thin, average or thick is useful information. If a cornea's thickness is less than 500 µm we need to be concerned because we know from the Ocular Hypertension Treatment Study that a thinner cornea is more likely to convert to glaucoma over time. (This can be at least partly attributed to the fact that a thinner cornea requires less force to flatten, making Goldmann readings underestimate the true pressure.)
If a cornea is thicker than 600 µm, the patient is probably in less danger. Thicker corneas tend to produce artificially higher readings, and the literature suggests that these individuals have a better prognosis. I've had patients with very thick corneas come to me with a diagnosis of ocular hypertension, taking two or three medications, but with no nerve damage or visual field loss. I tell them that their pressure is probably not as high as the tonometer indicates, and reduce their therapy. Of course, you still need to follow them expectantly, but you can be fairly sure that their true pressure is lower than what you're reading off the machine.
• A patient's relative change in IOP is useful information. Despite the fact that corneal biomechanical properties in a given patient may shift Goldmann readings higher or lower than the actual pressure, we can still use these measurements to gauge our treatment and its effectiveness. For example, if a patient measures 24 mmHg using Goldmann, we probably need to strive for a significant pressure reduction on the order of 25 or 30 percent—based on the patient's presentation of glaucomatous damage—and we can judge the effectiveness of the treatment in terms of the relative drop in pressure, whether or not the numbers are absolute.
• One CCT measurement may not be good for life. Currently, most ophthalmologists only measure a patient's CCT once. Aside from the possibility of short-term changes in thickness caused by hydration status or edema, we have data soon to be published in the British Journal of Ophthalmology showing that corneas can thin over time. We took CCT measurements eight years apart, and found they had thinned 1 to 23 µm, a statistically significant change.
Of course, statistical significance and clinical significance aren't necessarily the same; this level of change might not alter our recommendations. On the other hand, people can have glaucoma for decades, so it's probably worth measuring CCT again when considerable time has passed.
We've also followed some patients who've had trabeculectomies and found that their corneas thin over time. (Notably, endothelial function didn't change.) We haven't published the data yet, but it seems possible that lowering the pressure significantly can lead to thinning of the cornea, and consequently falsely low IOP measurements.
You should certainly remeasure CCT if the patient has had any kind of surgical intervention such as refractive surgery, which may alter both the CCT and the corneal biomechanics.
• Measure in the center. Studies have shown that there's about a 3-mm diameter area in the center of the cornea where thickness is fairly uniform. When you measure IOP or corneal thickness you need to be centered over the pupil, holding the probe perpendicular, to get an accurate measurement.
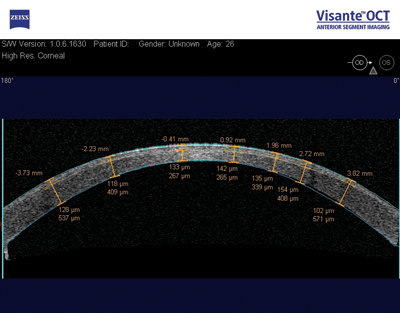 |
| CCT should be remeasured following refractive surgery. Here, a Visante anterior chamber OCT image (Zeiss) shows postop flap thickness and residual thickness of the stromal bed. Studies indicate that trabeculectomies, as well as the passage of time, may also affect corneal thickness. Mark Packer, MD, FACS |
• View IOP in context with other measures. IOP measurement is important, but we have a lot of other things to look at as well: the nerve; the visual field; OCT scans and other modalities. Pressure is just one aspect of the patient's overall situation. All the information at our disposal should contribute to determining the best treatment option for the patient.
Headed in the Right Direction
Researchers are now investigating the possibility that a thick or thin cornea may tell us something about the back of the eye. It's been noted that African Americans have thinner corneas than Caucasians, and often have more advanced glaucoma.2,3 So, a thinner cornea may indicate a thinner or more susceptible lamina cribrosa or optic nerve. Other research has found that ocular hypertensive patients with thinner corneas have thinner nerve fiber layers than ocular hypertensive patients with thicker corneas and healthy control subjects.4 There may be an anatomical correlation, but it's not well-established yet.
Clearly, we've learned a lot about the interaction of CCT and glaucoma during the past 30 years; hopefully it won't take another 30 to resolve the remaining issues. In any case, we know enough right now to be of real help to our patients, and the future looks even brighter.
Dr. Herndon is associate professor of ophthalmology in the Glaucoma Service at Duke University Eye Center in Durham.
1. Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J Cataract Refract Surg 2005;31:146-155.
2. LaRosa FA, Gross RL, Orengo-Nania S. Central corneal thickness of Caucasians and African Americans in glaucomatous and nonglaucomatous populations. Arch Ophthalmol 2001;119:23-27.
3. Shimmyo M, Ross AJ, Moy A, et al. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics, and African Americans. Am J Ophthalmol 2003;136:603-613.
4. Henderson PA, Medeiros FA, Zangwill LM, et al. Relationship between central corneal thickness and retinal nerve fiber layer thickness in ocular hypertensive patients. Ophthalmology 2005;112:251-256.




