Below is a review of cataract surgery challenges that range from the immediately obvious to the seemingly imperceptible. From trauma-induced issues decades in the making to a failure to achieve what seemed like easily attainable postop goals. Plus, a capsule tear in a shallow anterior chamber at the worst possible time. All of these dilemmas were eventually resolved. Here’s how.
Case #1:
Trauma-induced Cataract, 50 Years Later
The 69-year-old male suffered a battery explosion in his right eye in the 1970s, receiving only eye drops for treatment. The result: A pupil sphincter tear, zonular dialysis and, eventually, a traumatic cataract (See Figure 1). The patient was counting fingers at three feet by the time he visited Kevin M. Miller, MD, of the Stein Eye Institute at the University of California Los Angeles.
“This case called for a manual approach,” explains Dr. Miller. “I used phaco, instead of the femtosecond laser, my usual choice. But first I fashioned an inferotemporal Hoffman pocket where the zonules were missing so I could eventually suture a capsule tension segment to the sclera in the meridian with the greatest zonular loss.”
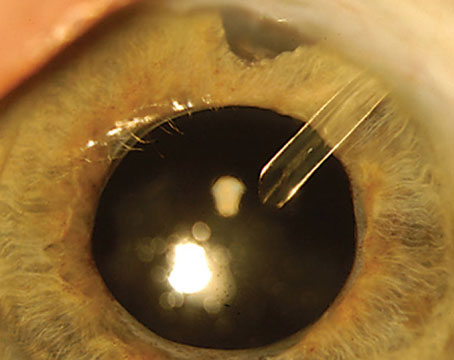
 |
| Figure 1. A slit lamp view of a patient’s eye reveals a dense cataract, zonulopathy and a pupil sphincter tear. Photo: Kevin Miller, MD. |
Dr. Miller then performed vitrectomies in the posterior and anterior chambers, first turning to a pars plana approach with the use of a diamond knife to create a sclerotomy. Once he’d amputated the vitreous connection between the front and back of the eye, he removed the vitreous in the anterior chamber, being careful not to capture the iris with the vitrector.
Next, Dr. Miller inserted a capsule retractor in the area of the zonulopathy at 4 o’clock. “I know some surgeons might’ve inserted up to four capsule retractors and/or iris hooks in a case like this,” says Dr. Miller. “But I was able to use a single capsule retractor, knowing that I could use more, if necessary.”
Divide and Conquer?
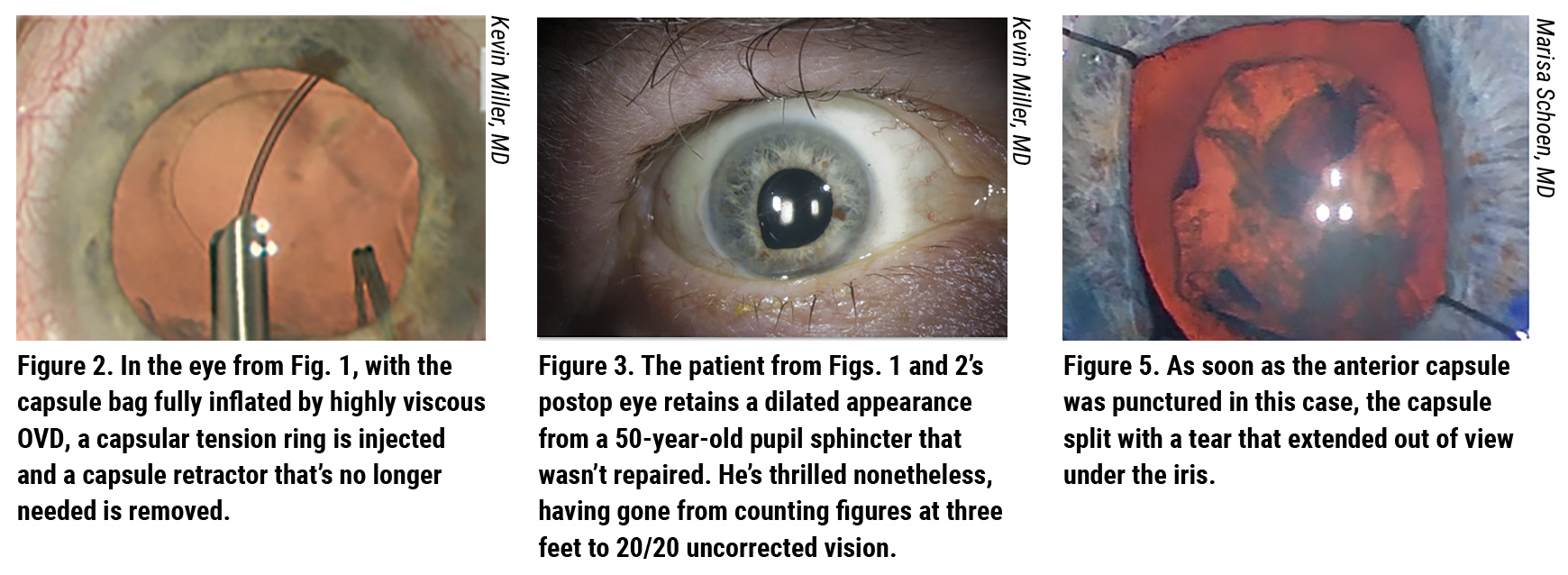
Rather than employing a chop or standard divide-and-conquer approach, Dr. Miller opted for an in-situ, divide-and-conquer technique (See Figure 2). “The nucleus wasn’t rotating at all, and the capsular bag remained stable,” he says. “I removed the nucleus and cortex without needing a capsule tension ring.”
Dr. Miller then injected a capsular tension ring and removed the capsule retractor (See Figure 3). “When injecting the ring, you need to make sure you fill the capsular bag with OVD, preferably a highly cohesive type,” he notes. “If you’re right-handed, you should inject as far to the right as possible, laying it out for 180 degrees and injecting toward the zonulopathy. After doing this, I had the support I needed. I removed the capsule retractor through the phaco incision.” A small amount of sub-incisional cortex remained. Dr. Miller gently polished the capsule—arguably a bold move, considering its vulnerability. “I just couldn’t help it,” admits Dr. Miller. “I had to get that stuff out of there.”
 |
Which Type of IOL?
In such a challenging case, many surgeons might’ve reasonably placed a three-piece IOL in the sulcus, knowing that they wouldn’t need to suture unless the lens decentered. “But I felt I could get away with a single-piece acrylic,” Dr. Miller says. “Yes, the zonules were weak, but I had the necessary support. If everything started to go south, I could’ve used the capsule tension ring to stabilize the eye. So I put the optic-haptic junctions of a single-piece acrylic IOL at 3 and 9 o’clock to reduce the chances of negative dysphotopsia. I retrieved the prolene sutures through the Hoffman pocket and tied them together.”
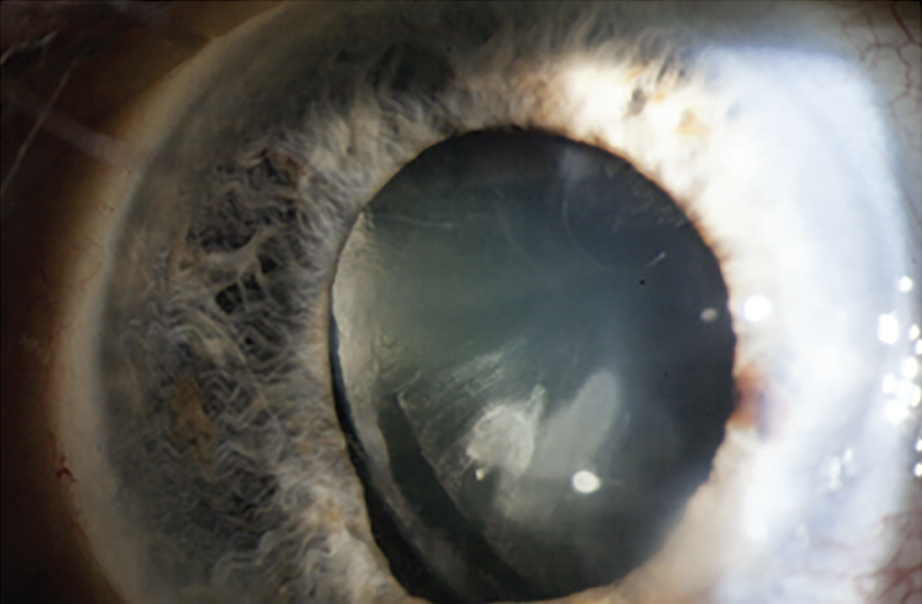
There was one last decision for him to make—to suture or not to suture the pupil sphincter where it had ruptured 50 years earlier. Dr. Miller decided to leave it alone. “I infused Miostat (carbachol 0.01% intraocular solution, Alcon) and let the pupil come back. I knew that, if necessary, once the capsule had fibrosed, I could go back later and suture the pupil sphincter.”
But it wasn’t necessary. “Cosmetically, the eye, with a dilated appearance, wasn’t ideal,” Dr. Miller admits (See Figure 3). “But the patient was very happy, seeing 20/20 uncorrected.”
Case #2:
What Else Could Go Wrong?
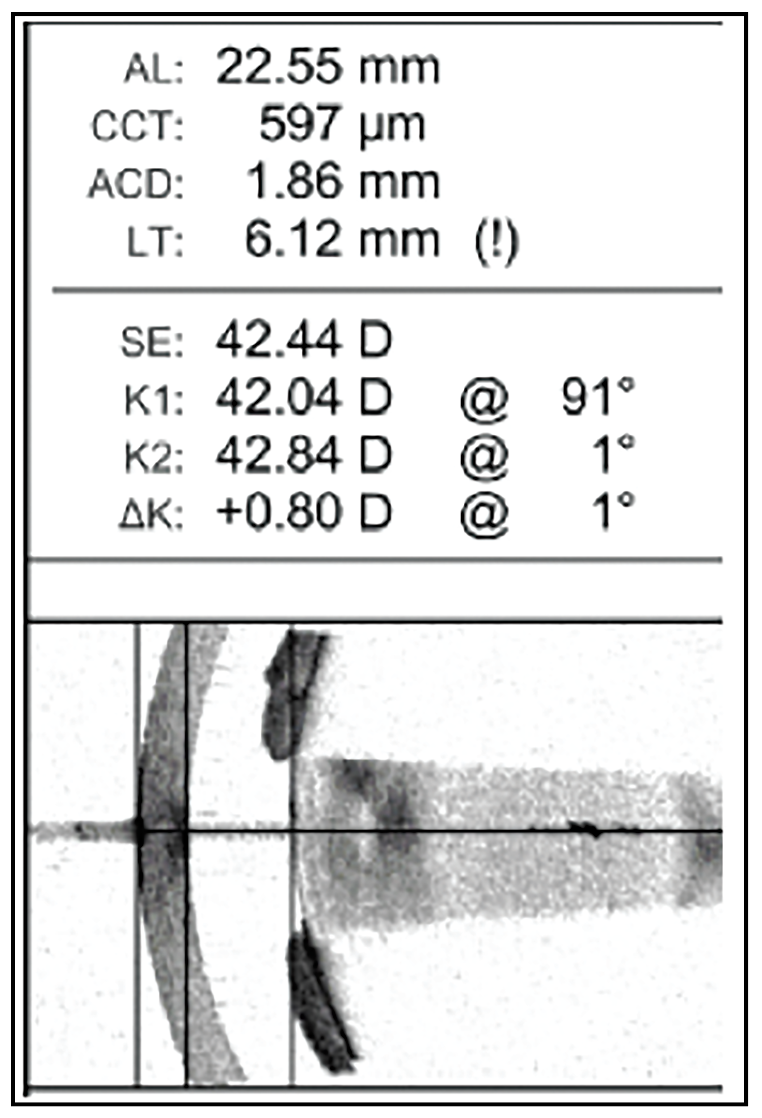 |
| Figure 4. Optical biometry reveals an anterior chamber depth of only 1.86 mm and a lens thickness of 6.12 mm. Photo: Marisa Schoen, MD. |
The early-career surgeon had her hands full with this case. A 60-year-old male with a history of proliferative diabetic retinopathy and anatomically narrow angles was referred for cataract evaluation. The preop exam revealed a small pupil with 360-degree posterior synechiae and a dense cataract. Optical biometry revealed an anterior chamber depth of only 1.86 mm and a lens thickness of 6.12 mm (See Figure 4).
“My biggest concern was the risk of an anterior capsule tear, given the big lens and shallow chamber,” explains Marisa Schoen, MD, a cataract and cornea surgeon at Ophthalmic Partners in Bala
Cynwyd, Pennsylvania.
Challenging Start
Before starting the case, she administered IV mannitol to dehydrate the vitreous. After releasing the posterior synechiae and placing iris hooks, she painted trypan onto the anterior capsule. She then filled the anterior chamber with Healon GV and initiated the capsulotomy with a cystotome. “As soon as I punctured the anterior capsule, it split with a tear that extended out of view under the iris (See Figure 5). So I switched to a can-opener technique to complete the rhexis.”
Before proceeding with phaco, Dr. Schoen avoided hydrodissection and lowered the bottle height to reduce pressure that could’ve worsened the tear. “Once an anterior capsule tear occurs, every effort must be made to reduce the risk of posterior extension,” she says. “I knew that posterior extension could lead to a worst-case scenario, with the whole lens falling into the vitreous cavity, requiring a vitrectomy and lensectomy.” After removing all of the nuclear fragments, she saw that, fortunately, the posterior capsule was intact and that the anterior capsular tear hadn’t extended posteriorly.
The next step was cortical cleanup. “In the setting of a tear, it’s important to pull cortical material toward—not away from—the tear to avoid stress that would cause the tear to extend, raising the possibility of vitreous prolapse,” says Dr. Schoen. “I also made sure I removed cortical material closest to the tear last to further minimize stress on the tear.”
Dr. Schoen notes that the patient had a dense posterior subcapsular cataract, which often leaves behind fibrous material that can be polished intraoperatively with the I/A handpiece. “Because of his compromised anterior capsule, I wasn’t as aggressive in cleaning the posterior capsule, recognizing that I could use a YAG laser to deal with it postop,” she explains. Dr. Schoen next placed a three-piece IOL in the capsule bag. “When placing an IOL in the bag in these situations, the haptics should be oriented 90 degrees away from the anterior capsular tear,” she notes.
Doing Very Well
Two months postop, Dr. Schoen performed a YAG capsulotomy. “The patient achieved an uncorrected distance vision of 20/50 that improved to 20/30 with a slightly myopic manifest refraction,” explains Dr. Schoen. “It was a pretty good result, considering how challenging the case had been, plus his underlying diabetic retinopathy. These cases can pose a lot of consequential challenges and complications, but if you recognize the potential for this preop and prepare yourself accordingly, a patient can still do very well.”
Case #3:
Contact Lens Confusion
Kendall Donaldson, MD, MS, recalls the frustration and horror she felt when she draped the patient’s left eye and placed the lid speculum, revealing a contact lens. The 60-year-old female patient, who’d undergone radial keratotomy 35 years earlier, had experienced a progressive decline in vision for two years. With severe irregular astigmatism (left eye worse than right), she’d worn both soft and scleral contact lenses. But she’d been instructed to discontinue all contact lens wear to prepare for surgery.
“When was your last contact lens wear?” asked Dr. Donaldson, medical director of Bascom Palmer Eye Institute in Plantation, Florida.
“Oh, I haven’t worn lenses in at least two months,” the patient replied, insisting, emphatically, that she wasn’t wearing a lens during her preop measurements three weeks earlier.
Surgical Dilemma
In sterile attire, Dr. Donaldson reviewed the patient’s measurements and asked for intraoperative aberrometry (ORA, Alcon) to be set up for the case, as she began the surgery with trepidation. She then learned the aberrometer was out of service, awaiting repair. “I thought, ‘This patient has 5 D of irregular astigmatism within the central 3 mm of her visual axis, so she will need a new contact lens after surgery anyway. I can’t leave her aphakic. Can I?’”
Surgery went perfectly, and Dr. Donaldson implanted the lens according to her preop measurements. But the patient returned postop day one with vision of 20/200. “I advised her to give it time to settle and we would watch her closely,” recalls Dr. Donaldson. One week postop, the patient was minimally better at 20/150, with pinhole vision of 20/30 in the left eye. One month postop, she was still extremely hyperopic (+4 +1.25 x 95). With some mild fluctuations, her refraction remained relatively stable, and she resumed soft contact lens wear in the left eye with a best corrected vision of 20/25.
About a year later, according to Dr. Donaldson, the patient returned to undergo cataract surgery in the right eye, achieving uncorrected visual acuity of 20/20-2. “Why is my other eye so bad and in need of a contact lens when this eye is now so good?” the patient asked.
“I pointed out that the left eye had eight-cut RK and the right eye only had four-cut RK, so the left eye had a much higher degree of irregular astigmatism,” recalls Dr. Donaldson. “And then the patient said, ‘Do you think it’s because of that contact lens you found during my first surgery?’ I had to admit to her that this had in fact made a bad situation worse.”
Dr. Donaldson agreed to perform a lens exchange in the troublesome left eye. “I wasn’t very confident,” she recalls, noting that the previous surgery had been two years earlier and that very severe irregular astigmatism continued within the visual axis. “I re-measured and re-calculated her lens in many different ways. I back-calculated from the lens in her eye. I used the Barrett Rx formula, and I discussed her case with several colleagues.” Dr. Donaldson eventually selected a final lens for the bag and, as a backup, a three-piece lens for the sulcus. Measurements ranged over 8 D. “I planned to use ORA (now functioning) to fine-tune my final lens choice,” she notes.
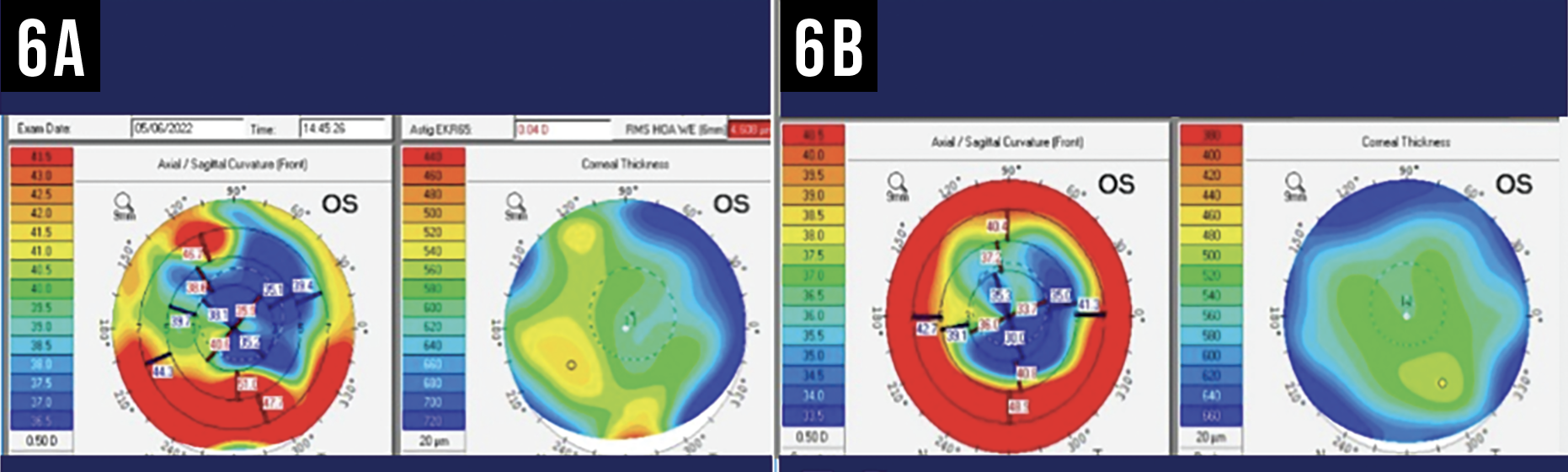 |
| Figure 6A and 6B. The left corneal topography map (6A) shows true, severe irregular astigmatism that was masked by a contact lens. The other map (6B) reflects true irregular astigmatism found in the same patient at a point in time when she wasn’t wearing a contact lens. Photo: Kendall Donaldson, MD. |
The Solution
Surgery began well. “However, the zonules appeared to be a little loose at the end of the lens dissection,” says Dr. Donaldson. “After considering a capsular tension ring, I placed the backup three-piece lens in the sulcus with a target of -0.75 D. Postop day one, the patient was 20/30-2 and extremely happy. By the time she returned one month postop, she could see both distance and near through her fortuitously multifocal cornea.” The patient functioned at both distance and near without glasses or contacts. “We bonded over our success—and our luck,” Dr. Donaldson recalls.
She now recommends canceling surgery if you ever encounter a contact lens on an eye in the operating room (See Figures 6A, 6B and 7). “The lens can alter the lens calculations by 4 D (as seen in this case) or potentially even more,” she points out.
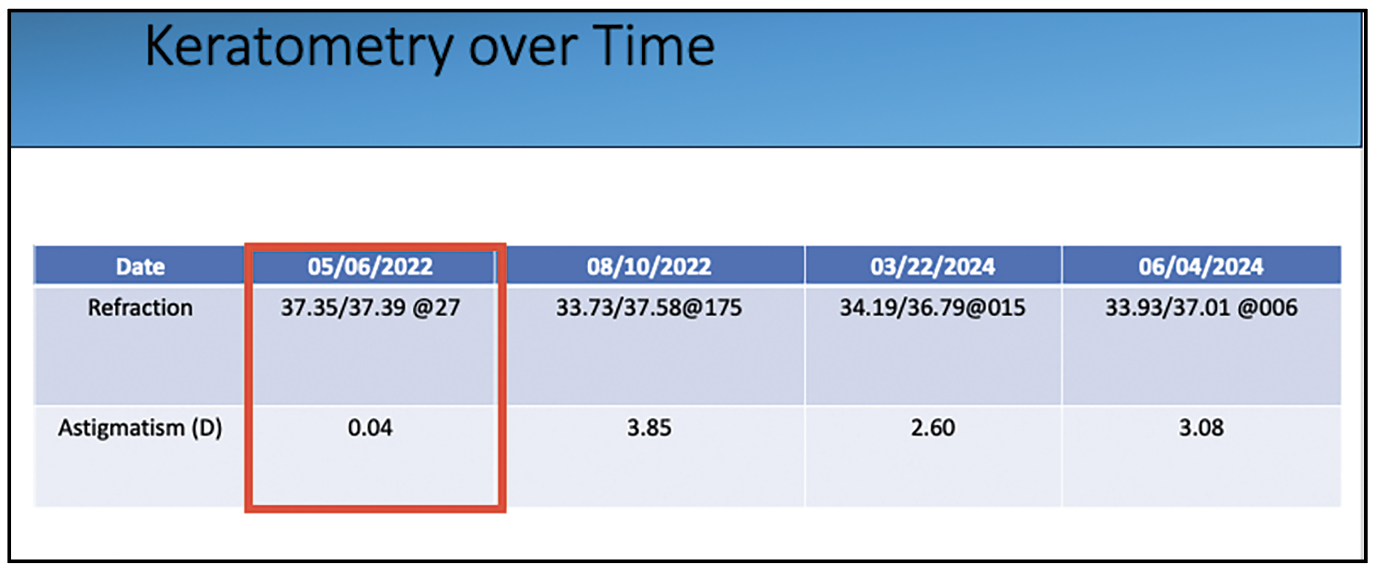 |
| Figure 7. Keratometry readings over time from the patient in Fig. 6A show the significant difference between this patient’s left-eye astigmatism readings and a left-eye reading affected by contact lens wear. |
Case #4:
If at First You Don’t Succeed . . .
Here’s an example of why patients with irregular corneas or a history of refractive surgery can sometimes be challenging, even when Light-Adjustable Lenses are used to enable non-invasive postop refinements.
The 67-year-old female patient was referred for possible cataract surgery at the Chu Vision Institute in Bloomington, Minnesota. She’d begun wearing progressive addition lenses full time a year earlier and had been experiencing difficulty driving at night. In 2001, she underwent LASIK for correction of myopia, and she’d been diagnosed with mild dry macular degeneration. Her preop topography showed a myopic LASIK pattern, with up to 2 D of astigmatism through the visual axis in her right eye (See Figure 8A) and up to 4 D of astigmatism through the visual axis in her left eye.
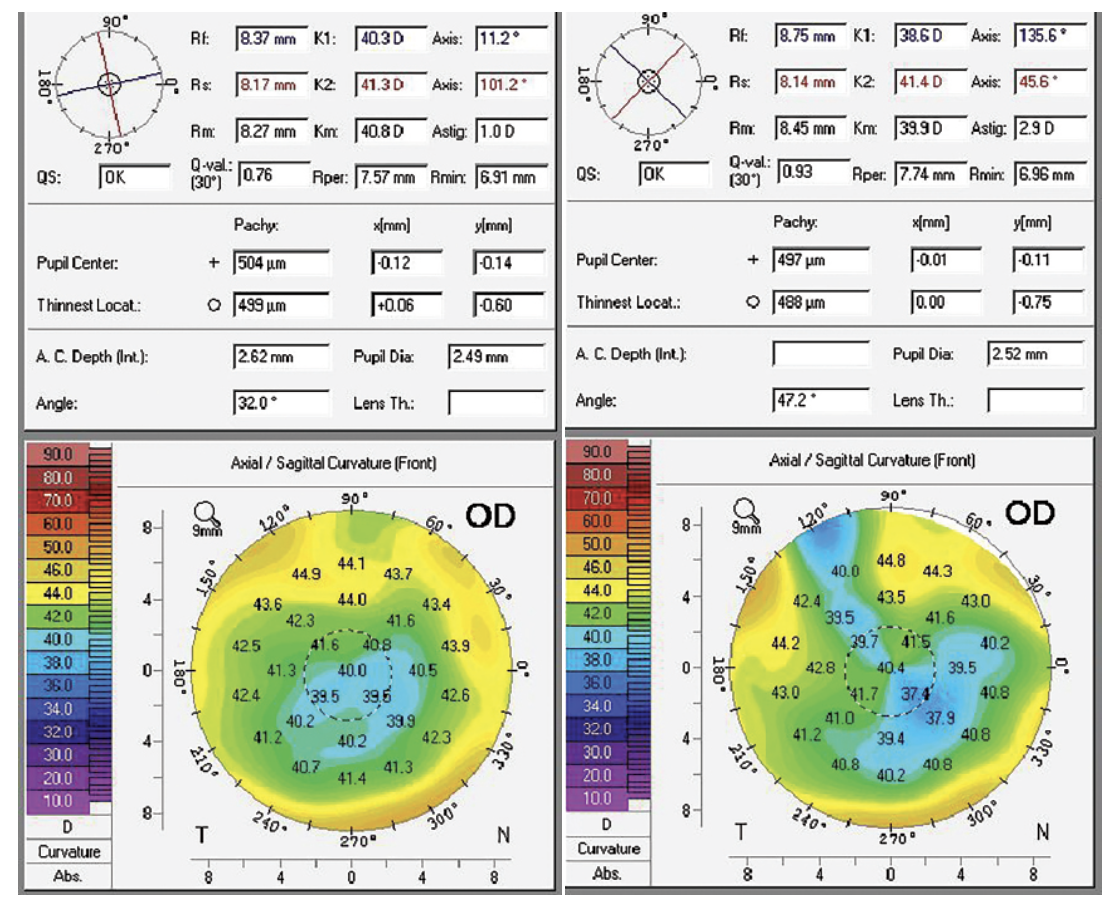 |
|
Figure 8A and 8B. 8A (left): Before receiving Light-Adjustable Lens implants, the patient’s preop topography shows a myopic LASIK pattern, with up to 2 D of cylinder through the visual axis in her right eye. There is up to 4 D of cylinder through the visual axis in her left eye (not pictured). 8B (right): The topography of the LAL implant patient after the patient’s first surgery in the right eye reveals expected flattening at the incision site and increased astigmatism through the visual axis. Photo: Y. Ralph Chu, MD. |
The patient had a BCVA of 20/20 in her right eye with plano sphere refraction and 20/25+ in her left eye with a correction of -3 + 0.50 x 173. With glare testing, her best corrected vision decreased to 20/50 OD and 20/70 OS.
The patient had unplanned monovision with the cataract progression, and she initially wanted to keep a distance target in both eyes. “The decision was made to proceed with cataract surgery with a Light-Adjustable Lens to give the patient a postop option of monovision,” says Ralph Chu, MD, the founder and medical director of the Chu Vision Institute.
Surgical Outcome
The patient underwent uncomplicated cataract surgery in both eyes with the implantation of Light-Adjustable Lenses. One week postop, she complained of blurred vision. “Her uncorrected vision was 20/40+ and >J10 in her right eye and it was correctable to 20/20 with a refraction of +1.75 +2.00 x 026,” explains Jessica Heckman, OD, vice president of clinical affairs and optometric residency director at Chu Vision. “The left eye was uncorrected at 20/50+ and >J10 but correctable to 20/20 with a manifest refraction of -1.50 +3.50 x 021. Her postop topography showed expected flattening at the incision site and increased astigmatism through the visual axis.” (See Figure 8B)
The refractive findings were concerning, considering the patient’s degree of hyperopia OD and astigmatism OS. “Her postop refractive error was pushing her lenses’ adjustability limits,” explains Dr. Chu. “We chose to perform an IOL exchange to reduce the refractive error to within a more adjustable range for her right eye. Incisional keratotomy or laser vision correction wasn’t possible.” No IOL exchange was planned for the left eye because of its possible use for monovision.
A 19.5 D LAL was exchanged for a 21.5 D LAL. At one week postop, the patient’s uncorrected vision measured 20/60—with a manifest refraction of 1.50 +2.75 x 035. “The patient said her vision felt slightly better, but she remained frustrated with her blurred distance vision,” says Dr. Chu. A prescription for glasses was written for functional vision until her right eye and refraction were stable enough for LAL adjustments.
Monovision Correction
The patient ended up needing two light-adjustment treatments on both eyes after the initial implantation. During this process, she found that she preferred more balanced distance vision in both eyes, instead of monovision. She ended up with uncorrected visual acuity of 20/20 and J3 in her right eye and 20/20 and J3 in her left eye. She also had J2 near vision in both eyes, with a +0.25 sphere manifest refraction in each. “She was very happy with her functional result,” Dr. Chu says.
“This case provides a good reminder that, even with the use of light-adjustable intraocular lens technology, patients with previous refractive surgery or irregular corneas may have a longer or more complicated journey after cataract surgery,” says Dr. Chu. In these cases, he recommends emphasizing to patients the possible need for additional surgery and the possible need to temporarily wear prescription glasses between surgeries.
Dr. Miller is a consultant for Alcon, BVI Medical, and Johnson & Johnson Vision. Dr. Schoen reports no relationships with ophthalmic companies. Dr. Donaldson has consulting relationships with Alcon, Johnson & Johnson Vision, Baush & Lomb, and Zeiss. Dr. Chu is an investigator for Zeiss, Bausch & Lomb, BVI Medical, Johnson & Johnson Vision, and Glaukos. He’s also a consultant for Zeiss, Bausch & Lomb, and RxSight, a maker of the Light Adjustable Lens. Dr. Heckman is a consultant for RxSight.