The approval was granted based on data from the COMPASS trial, a prospective, randomized, multicenter study that compared the pressure lowering in 505 patients who either underwent cataract surgery alone or cataract surgery plus implantation of the CyPass stent. Both the primary and secondary effectiveness endpoints were met; at 24 months, 73 percent in the CyPass group achieved a statistically significant decrease in IOP of more than 20 percent; 61 percent of the CyPass patients also achieved an IOP in the target range, between 6 and 18 mmHg—without medications—which was also statistically significant.
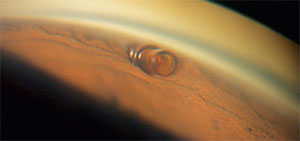 |
| The CyPass Micro-Stent does its work in a novel location: It’s placed through the angle between the scleral spur and the ciliary body into the supraciliary space. |
Adverse events included: BCVA loss of 10 or more letters at three months postop (8.8 percent for CyPass vs. 15.3 percent for cataract surgery only); anterior chamber cell and flare requiring steroid treatment 30 or more days after surgery (8.6 percent vs. 3.8 percent); worsening of visual field mean deviation by 2.5 dB or more (6.7 percent vs. 9.9 percent); an IOP increase of 10 or more mmHg 30 or more days after surgery (4.3 percent vs. 2.3 percent); and corneal edema 30 or more days after surgery, or severe in nature (3.5 percent vs. 1.5 percent).
“The FDA study for CyPass was performed in combination with phacoemulsification, and thus, this is where it will initially be used in clinical practice,” says Ike K. Ahmed, MD, FRCSC, assistant professor at the University of Toronto in Ontario, Canada, who participated in the clinical trial. “Many of us have also had experience using the CyPass as a standalone option, and we hope to see further data on that soon.”
Dr. Ahmed points out that this is another entry in the category of minimally invasive glaucoma surgery devices, or MIGS. “The suprachoroidal space is a natural drainage outflow tract that provides another MIGS alternative,” he says. “It’s exciting to see more options available for our patients. Further studies will help to elucidate differences between the growing MIGS options that are being approved.”
ASCRS/ASRS HORV Alert
Though the incidence is low after intraocular procedures, the American Society of Cataract and Refractive Surgery and the American Society of Retina Specialists joined forces to warn surgeons of the possible risk factors for and clinical signs of hemorrhagic occlusive retinal vasculitis. They also note that there seems to be an association between the use of intraocular vancomycin and the complication.
According to the alert, 22 cases of HORV have been identified, 14 of which were bilateral. In terms of timing, 12 of the cases occurred in 2015-2016, five in 2013-2014 and five before 2013.
“The difficult part about this complication is that it has occurred bilaterally in about half of the patients that were reported,” says Nick Mamalis, MD, professor of ophthalmology at the University of Utah’s Moran Eye Center and member of the HORV task force. “The sobering aspect is that it can have a delayed onset, meaning you can complete your first surgery, administer the antibiotic at the end of the case and the patient seems to do well initially. Then, a week to two weeks later, you do the second eye. After that eye’s surgery is complete, the HORV reaction manifests itself in the first eye. The time before onset shows a very wide range: Some have reported it as soon as a day postop, but in other cases that have been reported it went out as far as 26 days. So, in many cases, you may not know the patient will have this reaction until you’ve already done his second eye.”
The one thing all the cases had in common was the intraocular use of vancomycin, a connection that researchers are trying to parse out. “We really hadn’t seen anything like this before,” Dr. Mamalis recalls. “It was different from some of the other retinal issues that we’ve seen after intraocular surgery. For instance, in the past, there have been instances of patients getting some retinal toxicity from an injection of the incorrect dose of gentamycin, or surgeons who use a very high dose of cefuroxime that was improperly diluted and end up getting a retinal complication. This, however, was unique. HORV gave this pattern of vasculitis that truly behaved as a vasculitis. And, these cases were appearing up to two weeks after surgery without a toxic dose having been used. That aspect of it raised the suspicion that it had to be some sort of autoimmune reaction. The task force communicated with immunologists about it, and they have said that it’s consistent with what’s known as a Type III reaction, or what they call a leukocytoclastic vasculitis. That’s the pattern that it fits. HORV seems most consistent with that type of reaction.
“We were careful when issuing this alert, however,” Dr. Mamalis adds. “We don’t want to put out a blanket recommendation of, ‘Don’t use intracameral vancomycin,’ because there have probably been hundreds of thousands of cases of routine cataract surgeries performed with intracameral vancomycin that never had HORV. We’re careful not to condemn vancomycin. However, we want to alert physicians that this entity can occur and, though it’s exceedingly rare, when it does occur it can have very poor visual outcomes.” Twenty-two of the 36 eyes with HORV (61 percent), ended up 20/200 or worse, and eight (22 percent) were NLP. The task force notes that seven of the eyes (19 percent), received an additional bolus of intravitreal vancomycin for the treatment of presumed bacterial endophthalmitis postop. These eyes had especially bad outcomes, with 5/7 being NLP. This highlights the importance of spreading the word about HORV and avoiding treating it as an infection.
To help catch HORV, the task force highlights these characteristics:
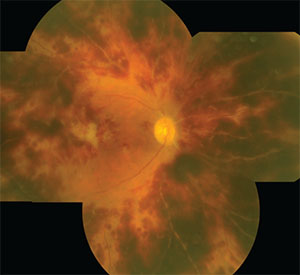 |
| A HORV eye, 18 days after cataract surgery, displays diffuse microvascular occlusion and extensive intraretinal hemorrhages. |
• occurrence after an intraocular procedure with normal undilated exam on postop day one;
• delayed onset of sudden painless decreased vision (range: one to 26 days postop; mean: eight days);
• visual acuity is often poor on presentation, but may be normal in mild cases of the complication;
• mild to moderate anterior chamber and vitreous inflammation, with no hypopyon;
• sectoral intraretinal hemorrhage in areas of nonperfusion;
• peripheral retinal involvement in all cases, with macular ischemia and whitening in advanced disease;
• sectoral retinal vasculitis and retinal vascular occlusion on FA, corresponding to areas of hemorrhage; and
• rapid progression to neovascular glaucoma.1
The alert notes that HORV might be misdiagnosed as a central retinal vein occlusion, but says that an ischemic CRVO is unilateral, usually presents on the first postop day when associated with cataract surgery, and is associated with diffuse, small intraretinal hemorrhages. In contrast, HORV is often bilateral, has a delayed onset, and often presents with large areas of intraretinal hemorrhage only in the sectors of retinal vascular occlusion. Cystoid macular edema and severe vascular dilation and tortuosity are features of CRVO but not of HORV.1
“Surgeons should continue to weigh the relative merits of prophylactic intraocular antibiotic use in preventing endophthalmitis against the additional knowledge that intraocular vancomycin is associated with HORV, a rare but potentially devastating disease,” Dr. Mamalis says. “In addition, surgeons using vancomycin prophylaxis with sequential cataract surgery should be aware that in addition to delayed onset of one to three weeks, HORV may be asymptomatic in the first eye and a dilated fundus exam may be the only way to detect it. The risk of bilateral HORV is therefore higher if close—or immediate sequential, same-day bilateral—surgery is performed. As alternatives to vancomycin, surgeons can use moxifloxacin or cefuroxime. Surgeons should have the issue of HORV enter into the consideration of how to approach antibiotic prophylaxis following routine cataract surgery.”
If a surgeon suspects or encounters HORV, once again, reconsider vancomycin. “The first thing to consider is avoiding intravitreal vancomycin if you’re considering HORV in a case that’s been diagnosed as endophthalmitis,” Dr. Mamalis says. “If you really aren’t sure that it’s endophthalmitis and suspect it may be HORV, pick another antibiotic. Also, look for other systemic syndromes if you’re searching for a potential viral cause. However, once you’ve determined that it’s HORV, you’ve got to be aggressive in terms of topical and systemic corticosteroids. Try to calm the inflammation. And, because these patients most likely will get significant glaucoma afterward—especially neovascular glaucoma—you want to consider using anti-VEGF treatments early, as well as consider doing panretinal photocoagulation early to take away the factors that can cause that secondary glaucoma.”
The ASRS has established a HORV registry to help track the complication that can be accessed at asrs.org. REVIEW
1. ASCRS/ASRS HORV Clinical Alert. http://www.ascrs.org/node/26101. Accessed 18 August 2016.



