Scanning laser polarimetry is a technique for assessing the thickness of the retinal nerve fiber layer in vivo. Though the technology has been available for several years, recent advances have resulted in improved imaging capabilities that may enhance clinicians' ability to identify and follow the progression of glaucoma. This article will describe the technology and the addition of the variable corneal compensation capability.
Background
The operating principle of the technology is the measurement of the retardation (phase shift) of a polarized laser light passing through tissue possessing the physical property of form birefringence. Form birefringence occurs in tissue that is composed of parallel structures, each of which is of a smaller diameter than the wavelength of the light used to image it.
Birefringence in the retinal nerve fiber layer (RNFL) arises from the microtubules contained within the individual nerve fibers. The greater the number of microtubules, the greater the retardation of the polarized laser light, indicating the presence of more tissue. Scanning laser polarimetry thus gives an indirect assessment of the thickness of the RNFL. The measurements obtained with this technique have been shown to be reproducible, and to be capable of differentiating patients with glaucoma from patients at risk or normal patients.
Corneal Complication
The RNFL is not the only structure in the eye that has form birefringence. The cornea, and to a lesser degree the lens, also have form birefringent properties. These tissues contribute to the total retardation signal. The GDx (Laser Diagnostic Technologies Inc., San Diego), the first commercially available scanning laser polarimeter, uses a proprietary compensator to remove the portion of the signal attributed to the anterior segment.
This compensator assumes the contribution of the anterior segment to be 60 nanometers in magnitude (the population average) at a slow polarization axis of 15 degrees nasally downward (the population mode). Work done at Bascom Palmer by David Greenfield, MD, and his coworkers using a slit-lamp mounted device to derive measurements based upon the 4th Purkinje image indicated that up to 30 percent of people have a slow polarization axis lying more than 10 degrees away from the assumed axis. Such people have been termed anterior segment "outliers."
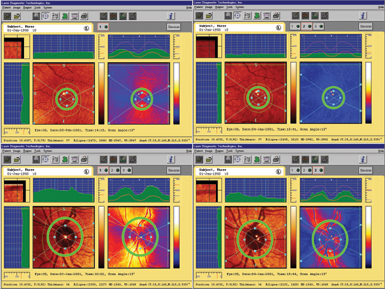 |
| Figure 1. An example of the effect of variable compensation on scanning laser polarimetry measurements of the peripapillary retinal nerve fiber layer. First, the macular image demonstrates a significant birefringence pattern when imaged using the fixed compensator set at 60 nanometers and 15 degrees nasally downward (upper left), due to residual uncompensated anterior segment birefringence. The resultant peripapillary measurements show very high values (lower left). Note the very bright colors on the retardation map and the height of the "TSNIT" plot at the top of the normative range. Setting the variable compensator to eliminate the anterior segment birefringence as determined from the macular image, in this case magnitude of 48 nanometers and slow polarization axis at 43 degrees nasally downward, eliminates the birefringence pattern in the macula (upper right) and reduces the peripapillary measurements (lower right). |
Further study in house at Laser Diagnostic Technologies, and corroborated by Robert Weinreb, MD and his coworkers, has shown that magnitude may vary from 0 to 120 nanometers in normal people. Even if someone had a slow polarization axis in the anterior segment that matched the compensator, a significant deviation from the assumed magnitude could result in over- or under-compensation.
The issue with the GDx, therefore, is that residual (incompletely compensated) anterior segment birefringence affects the measurements of retinal nerve fiber layer thickness obtained with scanning laser polarimetry if the patient is an anterior segment outlier.
For example, a patient with glaucoma could be under-compensated (not enough of the anterior segment signal removed, making the RNFL look "thicker") and appear normal (a false negative) while a normal patient could be over-compensated and appear to have RNFL loss (a false positive). This further affects sensitivity and specificity values by widening the confidence intervals for normal and glaucomatous eyes. Complete elimination of anterior segment birefringence should increase the overall accuracy of scanning laser polarimetry for determining RNFL thickness.
Spotting Outliers
How, then, can we determine who is an outlier and how can that information be used in the interpretation of scanning laser polarimetry results obtained from the GDx? The amount and orientation of anterior segment birefringence may be determined by obtaining a scan from the macula. Since the Henle layer is uniform and relatively thin compared to the peripapillary RNFL, an eye with completely compensated anterior segment birefringence will show a uniform "blue" appearance on a macular scan or a faint red "double-hump." The appearance of a "double-hump" or bright pattern in the macula indicates incomplete compensation. Measurements determined by this method have been shown to correlate with measurements obtained with the Greenfield/Knighton device based upon the 4th Purkinje image, with complete agreement with regards to axis. Magnitudes were highly correlated but different, attributed to differing wavelengths in the two devices and different optical pathways. It makes sense, therefore, to obtain a macular scan on every patient at least once in order to assess the degree of compensation. This is done by simply having the patient look straight down the center of the scan head. Focus and illumination are determined as for peripapillary measurements. A single measurement for each eye should be sufficient.
Interpreting the Results
How can the information obtained in a macula scan be used for interpreting the results of scanning laser polarimetry? At the present time, this information is useful only for a subjective analysis; no "correction" is currently available. If the macular pattern is uniform (as in Figure 1) or has a very faint "double-hump," the eye is well-compensated and the retardation map reflects information derived from the nerve fiber layer only.
Fortunately, this is the case in the vast majority of patients. If the patient is deemed to be an "outlier" as evidenced by a bright "double-hump" pattern in the macula, the vast majority of the time the retardation map will be showing values in the peripapillary RNFL that are usually higher than what they should be, with accentuated superior and inferior peaks. Usually the orientation of the "double-hump" in the macula will parallel the areas of brightest signal in the retardation map, easing interpretation of the scans that have strange patterns.
The most difficult outliers to recognize without quantifying the information in the macular scan are the ones that are "over-compensated" and have lower values than they should. I expect to see a very dark blue uniform appearance on the macular image in these cases, consistent with an anterior segment of low magnitude birefringence. These cases are rare.
You can also obtain clues regarding compensation just from the peripapillary images. Often, under-compensated eyes will have relatively high nasal and temporal values (See Figure 1), and reduction in modulation will be the key to identifying glaucoma damage. In fact, a study conducted by LDT comparing images obtained with the fixed compensator to those obtained with a variable compensator under development (to be used for "individualized" or custom compensation) has shown that while "thickness" values (superior maximum, inferior maximum, average thickness, ellipse average, superior average, inferior average and superior integral) may be higher with the fixed compensator, modulation parameters (symmetry, superior/nasal ratio, superior ratio, inferior ratio, maximum modulation and ellipse modulation) are not affected by compensation. Therefore, modulation parameters are the best measures of glaucomatous damage.
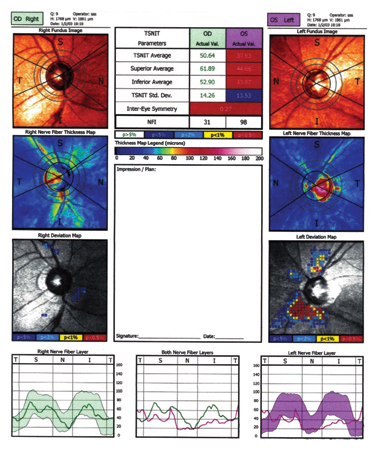
Figure 2. Printout from the GDx VCC. This patient has elevated Intraocular pressure and normal visual fields. The left eye in particular shows thinning of the RNFL as seen in the Deviation Map.
Interpretation
As with any clinical test, be it visual fields or scanning laser polarimetry, the last step in the interpretation is clinical correlation. If a scan doesn't make sense or appear to be consistent with the rest of the clinical picture, carefully analyze the macular image to identify compensation issues.
The two most common issues that come up are 1) the patient who has "advanced" glaucoma by clinical assessment who has a normal GDx exam (a false negative), and 2) the patient who has minimal to no glaucoma damage and has advanced loss on the GDx (a false positive).
If you encounter these types of cases, consider that false negatives may represent under-compensation, while false positives may be over-compensated. In any event, due to the high reproducibility of scanning laser polarimetry, all patients may be followed for change over time, with the first examination serving as the baseline.
Variable Compensator
As mentioned above, a variable anterior segment compensator has undergone clinical testing. Using information derived from a macular scan, custom compensation for each eye is available. Figure 7 is an example of measurements obtained with both the standard (fixed) compensator and the prototype custom compensation used in the GDx.
 |
| Figure 3. An experimental version of the variable corneal compensator. |
Unfortunately, a "hardware" custom compensator cannot be incorporated into the older model GDx. Users of this machine should perform macular imaging as discussed in the "Spotting Outliers" section and do a subjective assessment of compensation adequacy. It is hoped that a "software" compensation method will be available for the GDx and the old Access machines.
Variable corneal compensation (VCC) has been incorporated into the latest version of the GDx, the GDx VCC. A number of changes in the instrument have allowed this to take place. First, the old GDx Access platform was modified to allow incorporation of the compensator. The compensator (See Figure 3) consists of two optical retarders mounted in a rotatable housing. The retarders can be rotated relative to each other in order to vary the magnitude of the retardation. When the slow polarization axes of the retarders are orthogonal to each other, the net retardation is zero; when parallel, the net retardation is 120 nanometers. The housing can then be rotated to any axis, and thus can be set to neutralize the anterior segment birefringence.
The scan area on the GDx VCC has been increased to include the macula. In order to set the compensator, the first scan obtained (with the patient fixating on the internal fixation target) will use the macular information to determine the compensation needed. The compensator is then automatically set to neutralize the anterior segment birefringence, and the second scan will then determine the peripapillary RNFL values with the contribution of the anterior segment eliminated.
Other changes incorporated into the GDx VCC include new color scaling, a newly collected normative database, and a deviation map, similar to the probability plot used in automated perimetry. Figure 2 is an example of a printout from the GDx VCC. A number of investigators have suggested that custom compensation for the anterior segment increases the sensitivity and specificity of scanning laser polarimetry for differentiating normal eyes from those with glaucoma.
Dr. Choplin is a consultant for Laser Diagnostic Technologies.
Suggested Reading
1. Bour LJ. Polarized light and the eye. In: Charman WN, ed. Visual Optics and Instrumentation. Boca Raton, FL:CRC Press, 1991:310-25.
2. Choplin NT, Lundy DC. Differentiating patients with glaucoma from glaucoma suspects and normal subjects by nerve fiber layer assessment with scanning laser polarimetry. Ophthalmology 1998;105:2068-76.
3. Choplin NT, Lundy DC. The sensitivity and specificity of scanning laser polarimetry in the detection of glaucoma in a clinical setting. Ophthalmology 2001;108:899-204.
4. Garway-Heath DF, Greaney M, Roxier M, Caprioli J. Estimation and correction of the corneal component of retardation with the scanning laser polarimeter for glaucoma diagnosis [ARVO abstract]. Invest Ophthalmol Vis Sci 2000;41(4): S121 Abstract Nr 620.
5. Greenfield Ds, Knighton RW, Huang X-R. Effect of corneal polarization axis on assessment of retinal nerve fiber layer thickness by scanning laser polarimetry. Am J Ophthalmol 2000;129:715-22.
6. Hunter DG, Patel SN, Guyton DL. Automated detection of foveal fixation by use of retinal birefringence scanning. Appl Opt 1999;39:1273-9.
7. Villain MA, Greenfield DS, Knighton RW et al. Normative retardation data corrected for corneal polarization axis using scanning laser polarimetry [ARVO abstract]. Invest Ophthalmol Vis Sci 2001; 42(4):S135 Abstract Nr 716.
8. Zangwill L, Berry CA, Garden VS, Weinreb RN. Reproducibility of retardation measurements with the nerve fiber analyzer II. J Glaucoma 1997;6:384-9.
9. Zhou Q, Knighton RW, Boyett DC, et al. Variable corneal compensation on the assessment of the retinal nerve fiber layer thickness in eyes with corneal birefringence anomaly [ARVO abstract]. Invest Ophthalmol Vis Sci 2001;42(4):S16 Abstract Nr 90.