In recent years, along with visual fields, optical coherence tomography has become a mainstay for most practices that follow glaucoma patients. However, many ophthalmologists have come to believe that OCT is less useful for following progression when the disease is advanced. Instead, they shift their attention primarily to visual fields when patients reach that stage.
Recent studies are calling that conclusion about the usefulness of OCT into question, however, noting in particular that the fall-off in progression-monitoring capability is generally only true if you’re exclusively monitoring the circumpapillary retinal nerve fiber layer. Once you broaden your scanning to include the macular region, OCT may be very useful, even in advanced disease. Furthermore, even if you only monitor the optic nerve, some surgeons are questioning whether the drop-off in measurement capability is clinically significant for more than a few patients.
Here, surgeons with expertise in this area share their experience and offer advice on how to best use OCT when monitoring patients who have advanced glaucomatous disease.
Measuring the RNFL
Alexander T. Nguyen, MD, in private practice in Waterbury, Connecticut, and a clinical instructor at the Yale University School of Medicine, notes that the idea that OCT isn’t helpful for monitoring advanced glaucoma is a commonly held belief.
“I think this idea arose from a couple of studies, especially some spearheaded by the San Diego group and Felipe Medeiros, MD,” he says. “He and his group studied the relationship between visual field function, estimated ganglion cell counts and average retinal nerve fiber layer thickness. They noted that early in the disease OCT is very good at detecting changes that occur, while visual fields are not as helpful. From this they constructed what glaucoma specialists recognize as the expected structure-function curve. This model predicts that in advanced disease, the relationship reverses. Visual field testing becomes very sensitive to detecting changes in retinal ganglion cell loss, while change becomes more difficult to detect with OCT.”
Dr. Nguyen acknowledges that there’s some truth to this, explaining that the increasing measurement difficulty clinicians may encounter using OCT to monitor advanced disease can be understood in mathematical terms. “Imagine you have a million retinal ganglion cells,” he says. “If you lose 10 percent early on, that’s 100,000 ganglion cells lost. That’s going to look like a big change on OCT, while a visual field might not pick up any functional loss. But in advanced disease, you might only have 100,000 retinal ganglion cells left. Losing 10 percent would be a loss of only 10,000 cells—a small loss that OCT might not be able to pick up. On the other hand, functional loss at this point might be more readily detected by visual field assessment.”
Sanjay Asrani, MD, professor of ophthalmology at Duke University School of Medicine, director of the Duke Eye Center of Cary and head of the Duke Glaucoma OCT Reading Center in Durham, North Carolina, agrees that the problem isn’t a lack of change; it’s the amount of change. “The problem is that the scale at which it’s happening at that point in the disease isn’t properly represented on the OCT displays of most manufacturers,” he says. “That makes it extremely difficult for us to identify and measure the change once the nerve fiber layer reaches the floor effect level, which is about 35 to 40 µm.”
Dr. Nguyen points out, however, that it’s easy to overestimate the difficulty of following progression in advanced disease using OCT—even if you’re only monitoring the retinal nerve fiber layer. “Yes, OCT can become less sensitive at detecting changes as we approach the measurement floor for these instruments, but most patients with advanced disease are not yet at that level,” he says. “Most of our patients haven’t reached the point of having no-light-perception vision, where their nerve fiber layer is on its last fibers. That means that OCT can still detect changes in the majority of advanced patients.
“It also matters whether you’re looking at specific parts of the retinal nerve fiber layer,” he continues. “For example, Jonathan Myers, MD, at Wills Eye Hospital, presented a paper at the 2018 meeting of the American Glaucoma Society that showed that if you look at specific sectors of the circumpapillary retinal nerve fiber layer, you’ll find that some of them are still preserved, even in advanced disease. In other words, you can still detect changes in certain sectors. And that’s just one example. The point is, not everyone with advanced disease has reached the measurement floor, so clinicians shouldn’t arbitrarily abandon OCT because a patient’s disease is advanced.”
Monitoring the Macula
It’s also become clear that changes in the macula caused by glaucoma progression are different from the changes around the optic nerve, opening up another avenue for keeping OCT in the game. “Doctors have been primarily looking at the nerve fiber layer,” Dr. Asrani points out. “When that reaches the floor effect, it’s extremely difficult to identify small changes anymore. But at that point, the macular thickness and/or ganglion cell thickness can assist in monitoring even advanced glaucoma. In other words, if you know where to look, you can still use OCT to follow patients with advanced glaucoma.”
Dr. Asrani explains that in advanced glaucoma, the macular region still has residual thickness. “That’s partly because in normal eyes, the ganglion cells are stacked up about six layers deep at the macula,” he says. “As a result, the volume occupied by the ganglion cell layer and nerve fiber layer is considerable at that location. That’s what makes it possible to continue to observe changes there.”
Dr. Nguyen notes that monitoring the macula has become a hot topic. “It’s been hypothesized that macular parameters in glaucoma progress more slowly,” he says. “The idea is that glaucoma patients may reach the measurement floor in the macular region later than in retinal nerve fiber layer, if change is occurring at a slower rate.
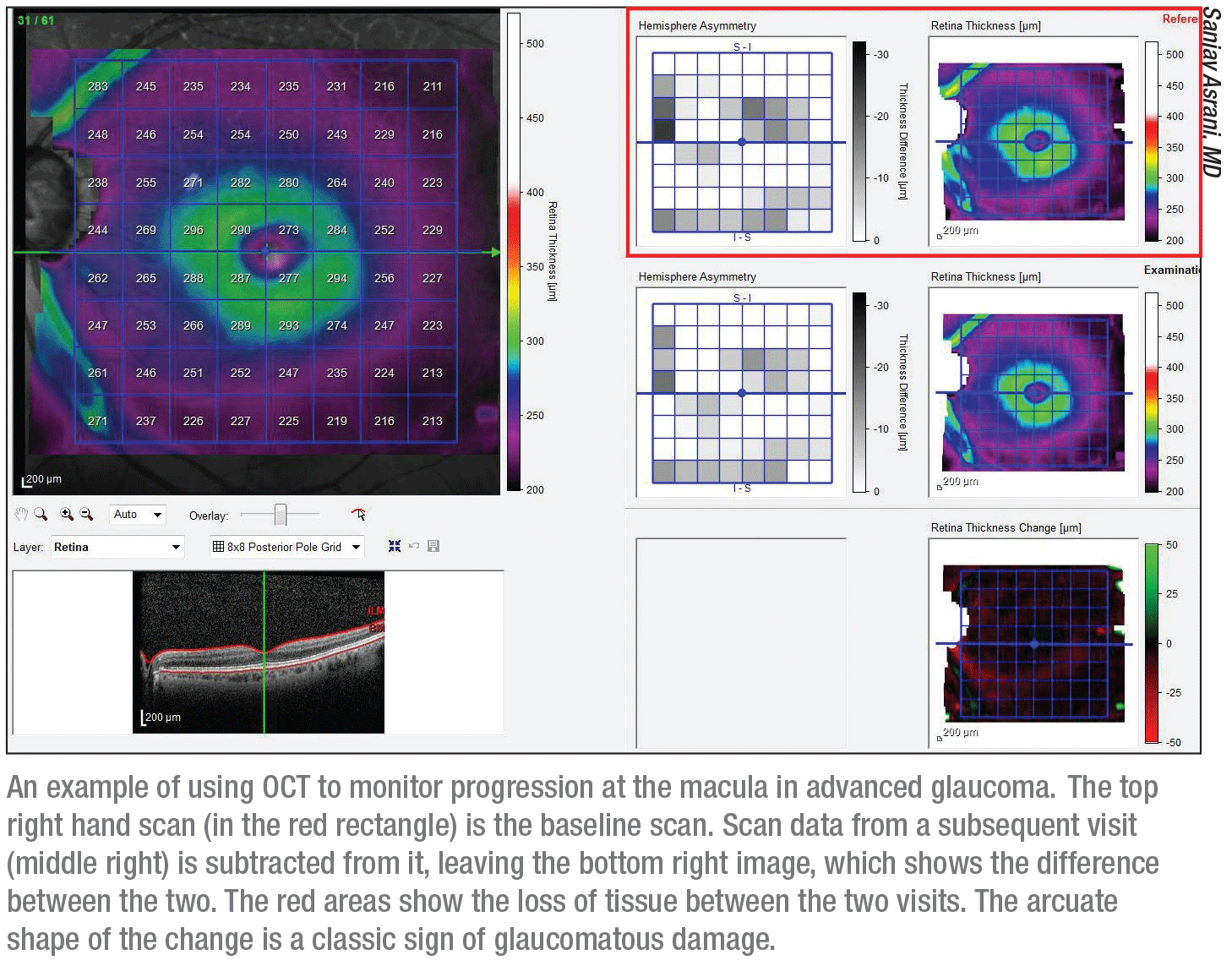 |
“Several papers have found evidence that macular OCT parameters may be better than the circumpapillary retinal nerve fiber layer for detecting changes later in the disease,” he continues. “For example, Joel Schuman, MD, recently published a paper that suggested that for patients with a retinal nerve fiber layer thickness less than 60 µm, he was still able to detect macular changes in his patients.1 Our group conducted a study that looked at both macular parameters and nerve fiber layer parameters, and we didn’t find this to be the case.2 However, we used a different definition of severe disease, and it’s possible that our study was too small; we may not have had enough patients for the data to confirm this finding.”
Dr. Asrani points out that it’s possible for the macula to reach a floor effect as well. “That can happen when the patient has a very small central island,” he says. “At that point the macula becomes extremely thin and you can’t follow progression beyond that point. Then, we’re forced to rely on a 10-2 visual field or subjective interpretation by the patient.”
Regarding the patient’s subjective analysis, Dr. Asrani explains that prior to this point, a patient won’t notice changes in vision caused by the disease unless he’s extremely vigilant. “In glaucoma, loss of vision is simply absence of vision,” he points out. “It’s not like a black area appears in your visual field. Even if patients have lost half of their peripheral vision, they typically can’t discern how much change is taking place until the disease reaches end stage. At that point in the disease, the patient can tell you whether or not he’s getting worse because there’s such a small amount of vision left.”
Strategies for Success
Surgeons offer these tips to help make the most of OCT monitoring of glaucoma patients:
• Monitor both the circumpapillary retinal nerve fiber layer data and the macular parameters, even in early disease. Dr. Nguyen encourages doctors to do this. “The information is complementary,” he says. “Some studies have been conducted to try to figure out if one is better for monitoring glaucoma progression, and there is some data suggesting that macular parameters might be a better predictor of visual field loss. Nevertheless, I think we should just follow them both. They both provide useful information.”
Dr. Asrani agrees. “When managing a glaucoma patient you should always monitor the macular thickness as well as the nerve fiber layer thickness,” he says. “If the change you see in the nerve fiber layer thickness is also present in the macula, that will give you more confidence that the changes in the nerve fiber layer are indeed real. So, you should do this from day one.
“Almost all machines have the capability of analyzing both the macula and retinal nerve fiber layer,” he adds. “It doesn’t add much testing time—only a few more seconds.”
• Establish a baseline; then look for macular changes in the shape of an arc. Dr. Asrani explains that the parameter you should monitor depends on the software of the OCT machine you use. “The software might give you the ganglion cell layer thickness or the thickness of the ganglion cell complex, which includes the nerve fiber layer thickness,” he says. “Some machines may give you the full thickness of the retina. Whichever hardware and software you’re using, establish a baseline and then see if change is happening in the macula.
“The macular changes are classically in an arc-like shape,” he notes. “That’s the typical hallmark of glaucoma. If it’s in the same region as where the nerve fiber layer has changed, it confirms that the change was caused by glaucoma.”
• Be alert for false positives caused by other macular problems. Dr. Nguyen points out that macular damage can also be caused by comorbid conditions, leading clinicians to erroneously believe that a patient’s glaucoma has gotten worse.
“When monitoring the macula for glaucoma, I’ve found that we have a lot of false positive readings because of the high incidence of other ocular conditions,” he says. “We’ve had quite a few patients with both glaucoma and macular degeneration or cystoid macular edema, whose macular parameters made them look like their glaucoma was getting worse. In reality, another problem such as macular degeneration was driving the change. So you have to remember that any macular damage you find could have been caused by another ocular disease.”
Dr. Nguyen says he hasn’t seen many papers addressing this potential source of confusion. “The problem is, when we do clinical studies, we want very clean data,” he explains. “For example, when we conducted our recent study, we excluded patients with macular degeneration, despite the fact that in our clinics many of our glaucoma patients have coincident macular pathology. There’s a paucity of data about how macular disease might result in false positives when monitoring the macula for glaucoma progression.
“I think this is a valid concern—especially if you’re seeing 100 patients a day and depending on an OCT algorithm to tell you who’s worsening,” he adds. “When you’re that busy, you may not have the time to review all the data yourself. You may falsely conclude that a patient’s glaucoma is getting worse, when the reality is that it’s the macular disease that’s causing the change.”
• Don’t assume a negative OCT finding is conclusive. “It’s possible to detect changes in severe disease using OCT, but we shouldn’t be falsely reassured if we don’t find evidence of change using OCT,” Dr. Nguyen points out. “It doesn’t mean that no loss is happening, because we need a lot more change in the OCT to detect progression late in the disease. So it’s possible that some of the these patients are progressing, even when the OCT readings appear stable—and it’s true that a visual field might be more sensitive in that situation.”
Still a Challenge
Dr. Nguyen says he’s sure that Dr. Medeiros would agree that OCT remains useful in advanced glaucoma. [Note: Dr. Medeiros was not available to comment for this article.] “In fact,” Dr. Nguyen says, “Dr. Medeiros has done some recent studies that have demonstrated this, including some interesting work that has tried to estimate where the structural measurement floor actually is.3,4
“I think the idea that OCT becomes useless in advanced glaucoma was a conclusion that became bloated and overused,” he continues. “In our work, OCT was more sensitive than visual field testing in detecting progression, even in advanced disease.” (Dr. Nguyen notes that some of the variability in reported sensitivities has to do with how different authors define advanced disease; he says his group used the clinical modification of the ICD.)
Despite acknowledging that OCT can still be useful in advanced disease, Dr. Nguyen warns clinicians not to ignore the challenges of doing so. “The basic issue is whether OCT is a good tool to monitor advanced glaucoma, and that’s a valid concern,” he says. “However, we don’t have a lot of great ways to monitor patients who have very advanced disease. Visual fields aren’t perfect in this situation either; field performance is highly variable. It becomes hard to tell who’s getting worse, and who’s just having a hard time doing the visual field test that day. Monitoring progression in advanced disease is still a challenging problem.” REVIEW
Dr. Asrani receives lecture honoraria from Heidelberg Engineering. Dr. Nguyen has no financial ties to any product mentioned.
1. Lavinsky F, Wu M, Schuman JS, Lucy KA, et al. Can macula and optic nerve head parameters detect glaucoma progression in eyes with advanced circumpapillary retinal nerve fiber layer damage? Ophthalmology 2018;125:12:1907-1912.
2. Nguyen AT, Greenfield DS, Bhakta AS, Lee J, Feuer WJ. Detecting glaucoma progression using Guided Progression Analysis with OCT and visual field assessment in eyes classified by International Classification of Disease severity codes. Ophthalmol Glaucoma 2019;2:1:36-36.
3. Belghith A, Medeiros FA, Bowd C, et al. Structural change can be detected in advanced-glaucoma eyes. Invest Ophthalmol Vis Sci 2016;57:9:511-8.
4. Bowd C, Zangwill LM, Weinreb RN, et al. Estimating optical coherence tomography structural measurement floors to improve detection of progression in advanced glaucoma. Am J Ophthalmol 2017;175:37-44.



