There’s no question that optical coherence tomography has had a profound impact on the field of ophthalmology. However, many surgeons still hesitate to use it unless it seems necessary.
 |
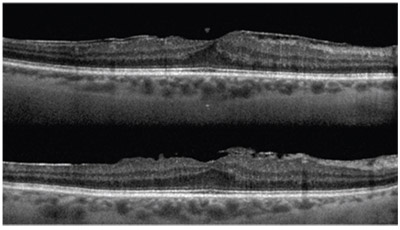 |
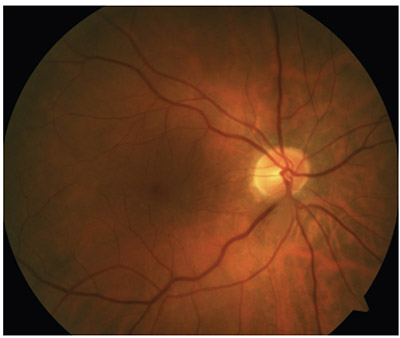
| Spectral-domain OCT can reveal macular problems that are otherwise invisible—problems that will be blamed on the cataract surgery if not caught beforehand. Here, a pre-existing epimacular membrane is revealed by OCT. |
One situation in which OCT is used infrequently is prior to cataract surgery on seemingly healthy patients. That may be a tactical error. It’s common to hear doctors talk about “refractive surprises” when discussing ways to improve cataract surgery outcomes. However, sometimes a poor outcome isn’t attributable to a refractive error; that’s why I’ve coined the phrase “visual surprises.” Visual surprises caused by macular problems that you failed to detect are just as upsetting to the patient as refractive surprises.
And while you can do a postoperative IOL exchange, you can’t do a macular exchange.
Performing an OCT scan of the retina is quick, safe and easy on the patient and staff, and there are many ways in which surgeons and patients stand to benefit from making the effort. Here are a few key reasons to use OCT to check the retina on every patient prior to cataract surgery.
• Many retinal pathologies can only be detected with spectraldomain OCT. Many types of retinal pathology are simply invisible through a microscope. Examples include: central serous retinopathy; vitreomacular traction syndrome; vitreomacular schisis; transparent epiretinal membranes; and some wet macular degeneration that hasn’t bled and doesn’t have any obvious choroidal neovascularization. These are invisible without an OCT scan. (In addition, the presence of the cataract may increase the difficulty of visualizing the condition of the macula.)
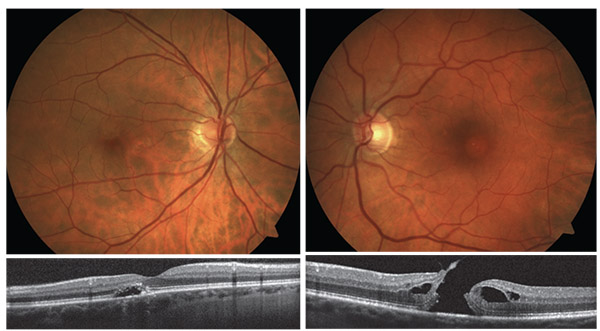 |
| Above, left: Pigment epithelial detachment. Right: A full-thickness macular hole. |
• If you missed a problem, everyone will assume your surgery caused it. If you find pathology in the macula or the peripheral retina before cataract surgery—particularly in the macula— it’s called a pre-existing condition. That’s how everyone, including the patient, will think of it. But regardless of the cause of that pathology, if it’s found after cataract surgery, it will be considered a complication—by the patient, by the referring optometrist and everyone else. That will be the case even if your cataract surgery didn’t cause it.
• In most cases, cataract surgery isn’t being performed by a retinal specialist. If you’re not a retina specialist, you’re less likely to be checking carefully for retinal problems that aren’t obvious. You’ll also have less training and knowledge of macular disease.
• Most preoperative exams are done in a hurry, with the focus on getting the refractive error correction right. The problem is: What if you make every possible measurement and the refractive outcome is perfect, but the patient can’t see well? That patient is not going to be happy. This is almost certain to happen to at least a few of your cataract surgery patients if you don’t use OCT before surgery.
• Patient expectations are at an all-time high. Small-incision refractive cataract surgery, femto, wavefront technology, intraoperative aberrometry, new technology IOLs, LASIK and marketing have raised patient’s postop visual expectations. If your patient has visual difficulty after your cataract surgery in this environment, he or she will be very unhappy indeed.
Making the Most of OCT
To get the most benefit from OCT in this situation, I recommend using the following strategies:
• Use OCT before cataract surgery for EVERY patient—not just premium IOL patients. When I first began suggesting the use of OCT to examine patients before cataract surgery, surgeons would come up to me at meetings and say, “Oh, you mean for premium IOL patients.” I’d say, “No, I mean for all patients.” This is about creating satisfied patients who can see well. Is that only an issue if the patient is paying extra? Surgeons would also ask who’s going to pay for it, and I’d point out that there’s no unit cost when using OCT. Then they’d ask, “How will we bill for this?” The truth is, I don’t know the answer to that question and I honestly don’t care. If you want to serve the patient and you want good outcomes, you need to examine the macula with OCT.
• Don’t do this using time-domain OCT. Time-domain OCT is obsolete. Spectral-domain or swept-source OCT is essential if you want to detect these vision-undermining pathologies.
• Don’t withhold OCT until something looks wrong at the slit lamp. Many surgeons don’t do an OCT unless the slit-lamp exam suggests that something is amiss. Since the pathologies we’re discussing can’t be detected during a slit-lamp exam, that’s a very bad premise. Even if you’re only dealing with a 1+ cataract, and you’re the world’s best retina expert looking with a 90-D lens at the slit lamp, a number of retinal pathologies will be invisible to you without OCT.
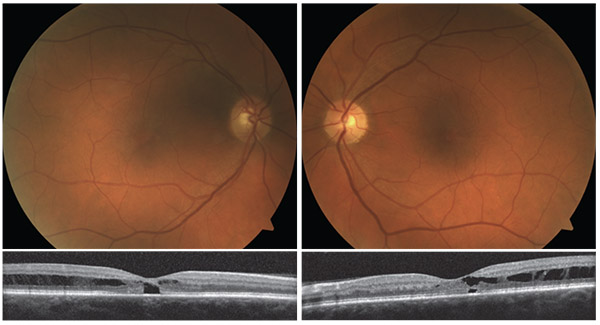 |
| Two examples of macular telangiectasia (Mac Tel) with macular schisis. |
• Beware of fragmentation between the operating surgeon and the preoperative examiner. If you don’t do the preop exam yourself, be sure that the doctor doing it scans the macula with OCT.
• Never use pseudo-color algorithms (i.e., color encoding), thickness maps or 3-D maps. This is not corneal topography. The aforementioned maps are generated using auto-segmentation algorithms, and the error rate is astronomical. The process of rendering them can cause crucial signs of pathology to be hidden.
• Look at every black-and-white slice yourself. I often hear surgeons say, “My EMR system allows the technician to import an image”—i.e., one image. If you’re doing this for diagnostics—as opposed to just for billing—you need to look at every slice of that scan yourself. Think of it this way: If you’re having a terrible headache and you go to the ER, do you want the X-ray technician to pick one film image and send it to the neuroradiologist to determine whether you have an aneurysm? Or do you want to have him look at all of the slices by scrolling up with his thumbwheel on the mouse?
If you want to be sure there’s no pathology present, look at every image yourself, using white images on a black background.
The Retina Factor
Here are a few other useful points to keep in mind when examining a patient before cataract surgery, and when working to avoid retina issues intraoperatively:
• If at all possible, measure axial length with optical low-coherence interferometry rather than ultrasound. If you have any macular pathology that causes elevation of the macula, such as fluid under the retinal membrane, macular edema, schisis or vitreomacular traction syndrome, the axial length measured by ultrasound will be inaccurate because the macula will appear to be forward of where it really is, causing an underestimation of the true axial length. If possible, measure the axial length with the Haag-Streit Lenstar or the Zeiss IOLMaster, which use optical low-coherence interferometry. They measure from the pigment epithelium, not the surface of the retina.
• Use the best tools when you’re examining at the slit lamp. To maximize your ability to catch a problem at the slit lamp:
— Use a 78to 90-D lens.
— The optimal view will be achieved if you use a plano fundus contact lens with anti-reflective coating.
— Blue-light autofluorescence is optimal for visualizing geographic atrophy in macular degeneration and cases of pre-existing central serous retinopathy.
Tools that are less helpful for macular evaluation before cataract surgery include angiography and ultra-widefield imaging, which has inadequate resolution for this purpose.
Be sure to dilate the eye and perform a careful examination of the peripheral retina using indirect ophthalmoscopy. Refer the patient to a retinal specialist if you find anything suspicious.
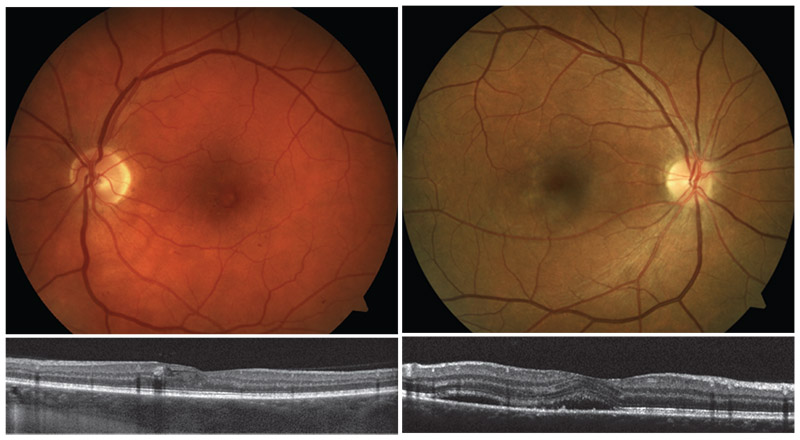 |
| Left: Early nonproliferative diabetic retinopathy. Right: Central serous chorioretinopathy. Macular serous detachment is more pronounced when visualized on OCT. |
• Don’t interrupt the patient’s anti-VEGF injection schedule because of cataract surgery. There’s no longer any reason to try to “stabilize” diabetic macular edema, wet AMD or retinal vein occlusion before cataract surgery. The surgery and the anti-VEGF protocol should proceed in parallel.
• Schedule cataract surgery midway between anti-VEGF injections. For example, if the patient is receiving injections on the first of the month, schedule the cataract surgery for the 15th of the month. Why? Because if you have an inflammatory response—or worse yet, endophthalmitis or hemorrhagic occlusive retinal vasculitis (HORV)—and you did the injection on Monday and the cataract surgery on Tuesday, from an epidemiology perspective, you won’t know which caused the inflammation or endophthalmitis. It’s far better to separate them by doing the cataract surgery midway between the injections. If any problem occurs, it will be clear where to look for the explanation.
• If you find any macular disease, avoid implanting a multifocal IOL. Multifocal IOLs decrease contrast sensitivity and should be avoided in most patients with any macular disease. This includes patients with significant drusen (intermediate drusen or placoid drusen), hyperpigmentation and/or wet macular degeneration. The risk of geographic atrophy and neovascular macular degeneration increases with each decade of aging. For that reason, placing a multifocal IOL in a healthy 55-year-old with intermediate drusen is not a good idea.
• Don’t confuse the presence of drusen with macular degeneration. Because of the way billing and coding works, there’s a tendency for doctors to tell patients that they have macular degeneration when in fact they just have drusen. That’s like telling every fair-skinned Swede that he has a melanoma. Yes, the presence of drusen does have consequences, but those consequences don’t necessarily include macular degeneration. Drusen don’t cause macular degeneration; they are simply associated with it. It’s far better to tell such a patient that he’s at risk for AMD, not that he has AMD. (For what it’s worth, a finding of many large, placoid, confluent drusen indicates a greater risk than finding a few fine drusen. Also, drusen in the macula are of much more concern than extramacular drusen.)
Of course, if your patient is 50 years old and has a lot of drusen, you definitely should not implant a multifocal IOL that’s going to decrease contrast sensitivity in that eye. But don’t make the mistake of telling the patient that he has age-related macular degeneration just because you find drusen on the retina.
• Don’t worry about cataract surgery making macular degeneration or diabetic macular edema worse. There is no relationship between post-cataract-surgery CME and AMD, DME or epimacular membranes. You shouldn’t modify your strategy for preventing CME because you find drusen, or because the patient has macular degeneration or an epimacular membrane. The only patients who need to be treated as being at high risk of CME are patients with inflammatory conditions such as pars planitis or sarcoid uveitis.
There was a time when many surgeons believed that cataract surgery caused macular degeneration to progress. But the Age-related Eye Disease Study showed that in more than 4,000 patients, cataract surgery did not make macular degeneration worse.1 In addition, numerous people still believe that cataract surgery makes DME worse, but the evidence doesn’t support that; cataract surgery just sometimes causes inflammatory cystoid macular edema that’s interpreted as DME in a diabetic context. (Similarly, epimacular membranes do not cause more CME; the increase in thickness they can cause on OCT is sometimes misinterpreted as being CME. Also, vitreomacular traction syndrome does not make macular degeneration worse. They are unrelated conditions.)
• If the eye has an epimacular membrane, the order in which the surgeries are performed should depend on the grade of cataract. If the patient has a significant posterior subcapsular cataract of 3+ nuclear sclerosis, proceed with phaco and IOL implantation using the axial length— measured from the RPE—before performing macular surgery. If the eye has 1 to 2+ nuclear sclerosis, the macular surgery should be performed before the cataract surgery.
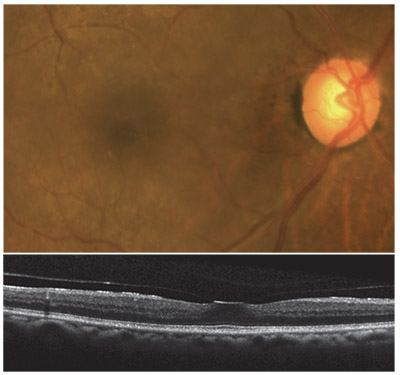 |
| Vitreomacular adhesion with mild traction. |
Better Safe than Sorry
“Refractive surprises” aren’t the only reason you can end up with an unhappy patient after cataract surgery. Performing an OCT on every single patient preoperatively—not just premium IOL patients—will prevent “visual surprises.” Doing this is good for your patients and patient satisfaction, and there’s no cost other than minor labor costs. Plus, it only takes a couple of minutes to accomplish. There’s really no downside to it.
Then, if you find macular pathology—pathology you wouldn’t otherwise have discovered—you can send the patient off for a proper retinal evaluation and treatment before you perform your cataract surgery. Preventing visual surprises will lead to a greater number of happy patients and better outcomes. So make a retinal OCT a standard part of your pre-cataract-surgery protocol. REVIEW
Dr. Charles is founder of the Charles Retina Institute in Memphis, and one of the world’s leading vitreoretinal surgeons.
1. Forooghian F, Agrón E, et al; Age-Related Eye Disease Study Research Group. Visual acuity outcomes after cataract surgery in patients with age-related macular degeneration: Age-related eye disease study report no. 27. Ophthalmology 2009;116:11: 2093-100.



