OCT is analogous to ultrasound B mode imaging, except that it uses light instead of sound.2 A beam of light is directed at the retina and the echo delay and magnitude of backreflected and backscattered light is measured, giving axial information similar to an ultrasound A scan. The light beam is then scanned in the transverse direction to generate a cross sectional image similar to an ultrasound B scan. OCT images can be displayed in grey scale or false color and represent a cross section through the tissue.
Although OCT is analogous to ultrasound, the technology of OCT is radically different. OCT imaging was challenging because the speed of light is one million times faster than the speed of sound. For example, light from the moon arrives at the earth after about two seconds. However, light traveling a distance of 10 um, about the diameter of a cell, arrives after only 30 femtoseconds (1 femtosecond = 10-15 of a second). The direct electronic detection of these high speed echoes is impossible and this type of high resolution time measurement can only be performed using correlation techniques. OCT measures the echo time delay of light by using interferometry, correlating echoes of light from the retina with light which has traveled a known reference path delay.
OCT was developed by our collaborative group at the Massachusetts Institute of Technology in 1990 and was first reported in 1991.2 The technology for OCT was made possible by advances in the fiber-optics and photonics industries which occurred during the 1980s. The first studies of OCT demonstrated ex vivo imaging of the retina and atherosclerotic plaque. Figure 1 shows the first published OCT image depicting the retina ex vivo and corresponding histology. In vivo retinal imaging was demonstrated in 1995.
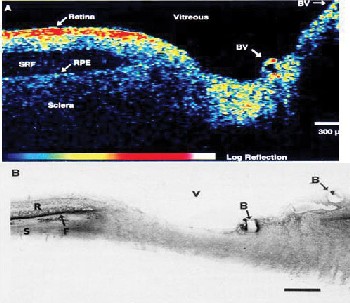 |
| Figure 1. The first OCT image of the retina ex vivo and corresponding histology. Note the detachment of the retina, common in post-mortem eyes. |
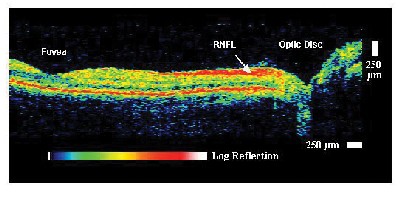
Figure 2 shows an example of an early OCT retinal image.3 This image has 10-µm axial resolution and demonstrates the ability to visualize retinal morphology including foveal contour, optic nerve head contour, and the retinal nerve fiber layer. The first clinical studies of OCT were performed by our group at the New England Eye Center of the Tufts University Medical School.3,4 These studies helped to establish the indications for OCT imaging as well as to develop OCT examination protocols.
|
|
| Figure 2. Example of an early OCT image of the normal retina in vivo. The retinal nerve fiber layer which emanates from the optic disc and decreases in thickness toward the macula can be clearly visualized. |
In order to have a clinical impact, a new technology must be developed by industry. We transferred OCT technology to Carl Zeiss Meditec (then Humphrey Instruments) in the early 1990s, and the first commercial OCT instrument, OCT1, was introduced into the ophthalmic marketplace in 1996. A third-generation OCT instrument, the StratusOCT, or OCT3, was introduced by Zeiss in 2002. The StratusOCT achieves a fivefold increase in imaging speed, enabling higher pixel density images. Extensive clinical studies performed by many outstanding research groups internationally have helped to make OCT a standard of care in ophthalmology. OCT has proven useful for the diagnosis and monitoring of glaucoma, diabetic retinopathy, age-related macular degeneration, macular holes, epiretinal membranes, and many other retinal diseases.5
Ultrahigh resolution OCT
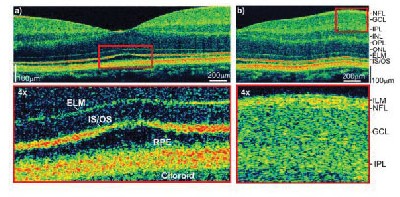
Fundamental research in OCT technology is continuing and yielding many exciting advances. Considerable effort has been focused on improving image resolution. The axial image resolution in OCT depends upon the resolution with which echo time delays of light can be measured. This axial image resolution is determined by the bandwidth of the light source used for imaging. Standard resolution OCT systems use superluminescent diode light sources which have bandwidths that support 10-µm axial resolution imaging. However, using advanced femtosecond laser light sources, axial image resolutions can be dramatically improved. Ultrahigh resolution (UHR) ophthalmic imaging was reported in 2001.6 Figure 3 shows an example of an ultrahigh resolution OCT (UHR-OCT) image with 3 µm axial image resolution. UHR significantly improves image quality and enables definitive visualization of individual retinal layers. Clinical investigations of UHR OCT are currently under way at the New England Eye Center (NEEC) and by collaborators at the Medical University of Vienna in Austria.
|
|
| Figure 3. Ultrahigh resolution OCT improved axial image resolution to 3 um, a factor of three to four times finer than standard OCT. With UHR OCT, individual layers of the retina can be visualized. |
|
|
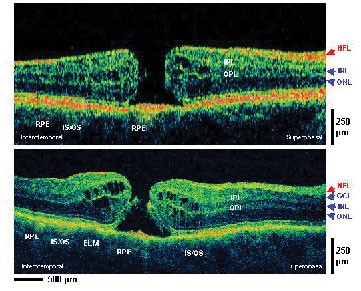
| Figure 4. UHR OCT improves the visualization of retinal pathology compared to standard resolution OCT. The structure of the photoreceptors including the external limiting membrane and the boundary between the inner and outer segments can be clearly visualized in this example of a stage three macular hole. |
Although currently limited to research use, UHR OCT has also provided important information on the interpretation of standard resolution clinical OCT images. One surprising finding was that the boundary between the inner and outer segments of the photoreceptors, which could be visualized using UHR OCT, had been widely misidentified in earlier, standard resolution OCT images.7,8 The limitation of UHR OCT is that it currently requires expensive and specialized laser technology which, although a powerful tool for research, is not feasible for general use in the ophthalmology clinic. With continuing advances in the fiber-optics and photonics industries, however, technology for new light sources is rapidly improving. New, high-performance, compact and low-cost superluminescent diode light sources for OCT imaging promise to enable improved axial image resolutions for clinical applications in the future.9
High-Speed, UHR OCT Using Spectral/Fourier Detection
In addition to improvements in resolution, there have also been considerable research advances in improving imaging speeds. High-speed imaging enables high pixel density, high definition OCT imaging. High speed also dramatically reduces eye motion artifacts, enabling more accurate measurements of retinal and optic disc topography. In addition, large numbers of images can be acquired to improve coverage of the retina and to avoid missing focal pathologies. Three dimensional OCT (3D OCT) imaging will ultimately become possible.
Recent advances in OCT technology enable a tenfold to one hundredfold increase in imaging speed. Measurement techniques known as spectral/Fourier domain detection enable dramatic improvements in sensitivity and speed for measuring echo time delays of light.10-15 Standard OCT techniques measure light echoes from a given time delay and scan the time delay in order to measure an axial scan. In contrast, spectral/Fourier detection can measure all of the light echoes from all time delays simultaneously. Spectral/Fourier detection detects light echoes by using an interferometer, spectrometer, and high speed CCD camera. The interference spectrum of the light is detected and digitally processed to construct the fourier transform, hence the name "spectral/Fourier" detection.
Spectral/Fourier detection was first demonstrated a decade ago.16 Only with recent advances in high-speed, digital CCD technology, however, a by-product of the digital photography industry, has this approach achieved the high- performance levels necessary for detecting the very small levels of backreflected and backscattering light from the retina. The first in vivo retinal imaging using OCT with spectral/Fourier domain detection was reported in 2002.10 Several groups demonstrated spectral OCT systems with acquisition speeds of 15,000 to 30,000 axial scans per second, factors of nearly 100x faster than the 400 axial scans per second imaging speed of standard OCT.11-14
Figure 5 shows an example of a high speed UHR OCT image using spectral/Fourier detection.14 The axial image resolution is ~2 µm and a high transverse pixel density image with 3,000 axial scans (transverse pixels) can be acquired in 0.150 seconds. This contrasts with standard OCT images which have 512 axial scans (transverse pixels) and are acquired in ~1 second. The high pixel density yields a "high-definition" OCT image. Like high-definition television, these images improve clarity. High definition OCT images can be zoomed and enlarged to examine fine details of retinal morphology. Figure 5 shows an enlargement of the photoreceptor layer of the normal fovea. The external limiting membrane is clearly visible as well as the junction between the inner and outer segments of the photoreceptors. The outer segments of the rods are thicker in the fovea, in agreement with expected normal retinal morphology. Figure 5 also shows an enlargement of the inner retina in the parafoveal region. The retinal nerve fiber layer is well visualized with its borders clearly demarcated. The ganglion cell layer and inner plexiform layer are also visible. The extremely thin inner limiting membrane may also be visible.
|
|
| Figure 5. High speed, UHR OCT using spectral/Fourier detection dramatically improves imaging speeds making high pixel density, high definition OCT imaging possible. These images can be zoomed to display fine features in the retina. |
|
|
| Figure 6. High speed imaging promises to enable three-dimensional OCT imaging of the retina. This example shows renderings of retinal layers in the macular and the nerve fiber layer around the optic disc. These advances will permit the in vivo visualization of retinal structure in ways that were previously impossible to achieve without biopsy and histology. |
OCT imaging has continually improved in performance since its development more than 10 years ago. The degree of improvement can be appreciated by comparing the OCT images in Figures 1 to 5. Although image quality was limited at first, even the earliest OCT images provided a view of the retina which was not possible to obtain using any other non-invasive imaging technology. Fundamental research has yielded dramatic improvements in OCT image quality, providing higher resolution and higher definition images. These advances in OCT technology have been made possible by powerful developments in photonics and electronic imaging technologies, driven by the consumer market. Current, commercially available OCT instruments enable visualization of retinal pathologies and quantitative mapping of retinal and retinal nerve fiber layer thickness. Research OCT imaging systems push the limits of optical technology and enable ultrahigh resolution, 3-D viewing of retinal structure. The ability to image the architectural morphology of retinal pathologies represents a major advance, since it will be possible to visualize structural changes with at a level of resolution and comprehensiveness previously impossible to obtain in the intact retina. These advances promise not only to improve our understanding of disease pathogenesis, but ultimately to enable earlier and more sensitive diagnosis, to improve the monitoring of disease progression, and assessment of response to therapy.
Dr. Duker is director of the New England Eye Center and chairman of the Department of Ophthalmology at Tufts New England Medical Center. Dr. Fujimotoa a professor of electrical engineering and computer science, Department of Electrical Engineering and Computer Science and Research Laboratory of Electronics at MIT. Mr. Witkin is a research intern at the NEEC. Dr.Wojtkowski is a visiting scientist at NEEC and MIT. The authors gratefully acknowledge the contributions of Tony Ko and Vivek Srinivasan and support from National Institutes of Health RO1-EY11289-19, R01-EY13178-05, and National Science Foundation ECS-0119452, Air Force Office of Scientific Research, MFEL F49620-01-1-0186.
1. Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature Biotechnology 2003;21:1361-7.
2. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991;254(5035):1178-81.
3. Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Archives of Ophthalmology 1995;113:325-32.
4. Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995;102:217-29.
5. Schuman JS, Puliafito CA, Fujimoto JG. Optical coherence tomography of ocular diseases, 2nd edition. Thorofare, NJ: Slack Inc., 2004.
6. Drexler W, Morgner U, Ghanta RK, et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nature Medicine 2001;7:502-7.
7. Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Archives of Ophthalmology 2003;121:695-706.
8. Ko TH, Fujimoto JG, Schuman JS, et al. Comparison of ultrahigh and standard resolution optical coherence tomography for imaging of macular pathology. Arch. Ophthalmol 2004;111:2033-43.
9. Ko TH, Adler DC, Fujimoto JG, et al. Ultrahigh resolution optical coherence tomography imaging with a broadband superluminescent diode light source. Optics Express 2004;12:2112-9.
10. Wojtkowski M, Leitgeb R, Kowalczyk A, et al. In vivo human retinal imaging by Fourier domain optical coherence tomography. Journal of Biomedical Optics 2002;7:457-63.
11. Nassif NA, Cense B, Park BH, et al. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Optics Express 2004;12(3).
12. Leitgeb RA, Drexler W, Unterhuber A, et al. Ultrahigh resolution Fourier domain optical coherence tomography. Optics Express 2004;12:2156-65.
13. Cense B, Nassif N, Chen TC, et al. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Optics Express 2004;12:2435-47.
14. Wojtkowski M, Srinivasan VJ, Ko TH, et al. Ultrahigh resolution, high speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Optics Express 2004;12:2404-22.
15. Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol 2004;138:412-9.
16. Fercher AF, Hitzenberger CK, Kamp G, Elzaiat SY. Measurement of Intraocular Distances by Backscattering Spectral Interferometry. Optics Communications 1995;117:43-8.