 |
|
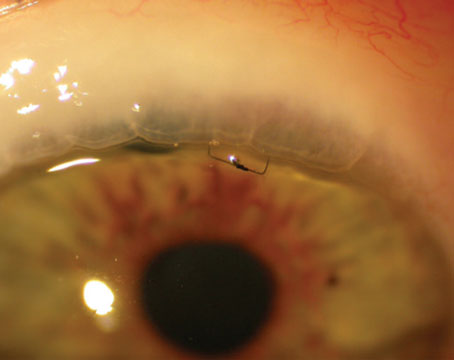
Implantation of a Baerveldt glaucoma shunt and surgical placement of the tube. |
Once we’ve decided that a patient would benefit from having a tube shunt implanted, we have to make a series of decisions. Those include which type of implant to use (valved or nonvalved), what size implant would be most appropriate, where to position the endplate on the eye, where to place the tube, how to deal with the delayed pressure control associated with using a nonvalved implant and what measures can be taken to minimize the risk of complications. Here, I’ll share some strategies that can help you make the best decisions for your patient and ensure the best possible outcome.
Selecting an Implant
As you know, tube shunts fall into two categories: nonvalved implants and valved implants. The latter incorporate a flow-restrictor into the design that limits the flow of aqueous through the device if the pressure drops too low. The Ahmed implant (New World Medical) is currently the only valved implant that’s in popular use. The Baerveldt (Johnson & Johnson Vision), Molteno (Molteno Ophthalmic) and the new Ahmed ClearPath (New World Medical) implants are all examples of nonvalved implants.
The first decision we need to make is whether a valved or nonvalved implant will best serve the patient we’re managing. As always, it’s best to base our decision on clinical trial data, in addition to our first-hand experience. Much of the information we have about the differences between these implants comes from two landmark clinical trials: the Ahmed-Baerveldt Comparison (ABC) Study and the Ahmed vs. Baerveldt (AVB) Study.
Clinical factors that would affect my choice of implant include:
• The patient needs a low postop pressure. The two major determinants of the final intraocular pressure after tube shunt surgery are the total surface area of the capsule around the endplate and the thickness of the capsule. There’s not much we can do to modulate the thickness of the capsule; that’s determined by the individual’s healing process. But we can influence the surface area, because an endplate with a larger surface area will produce a larger-area capsule around the endplate, and thus a lower pressure. In fact, the clinical evidence supports this.
The Ahmed glaucoma valve’s endplate has a smaller surface area than the Baerveldt and ClearPath devices, so using one of the latter two devices would make sense if you need to achieve a low pressure. For that reason, I tend to favor nonvalved implants like the 350 Baerveldt or the 350 ClearPath for patients who have advanced glaucoma or normal-tension glaucoma, where a low postop pressure is desirable.
• The patient has markedly elevated IOP. A valved implant makes sense for a patient in this situation because, unlike a nonvalved implant, you won’t need to temporarily restrict flow through the implant at the time of surgical implantation. This offers the advantage of allowing immediate pressure reduction, while minimizing the risk that the IOP will drop too low.
• The patient has a high risk of hypotony. I favor a valved implant like the Ahmed in patients at higher risk of hypotony because the valve mechanism helps to prevent the IOP from going too low. That’s particularly important in eyes that are prone to that complication, such as those with uveitic glaucoma; patients who have had prior cyclodestructive procedures; and also, in my opinion, patients who are significantly older—perhaps over the age of 85. The ciliary body makes less aqueous humor as we get older, so very elderly patients may be at a greater risk of hypotony.
• The patient is poorly adherent or intolerant of medication use. Larger-size endplates make sense for patients who are poorly adherent or have trouble tolerating drops. In both the ABC and AVB Studies, patients who received a Baerveldt implant required fewer glaucoma medications than those who received an Ahmed implant. Presumably this is related to the larger endplate providing greater efficacy.
• The patient has had a prior scleral buckling procedure. Implanting a tube shunt in patients who have a scleral buckle around the eye can be tricky. I’ve found that an implant with a lower profile, like the nonvalved Baerveldt or the new ClearPath, can be easily positioned over the buckle. That may reduce the likelihood of migration and erosion of the device.
• Where you’re placing the endplate. Most implants are placed in the superotemporal quadrant, but occasionally there’s a reason to place it elsewhere. My preference for a fallback location is the inferonasal quadrant. (A more detailed discussion of inferonasal placement is provided later in this article.)
When inferonasal placement is indicated, I tend to favor a nonvalved implant, although you can put a valved implant inferonasally if necessary. A nonvalved implant like the Baerveldt has an endplate with anterior-posterior dimensions that make it less likely to encroach on the optic nerve, given that the optic nerve inserts nasally. That’s of particular importance in eyes that are small, such as nanophthalmic eyes, and in children. It also has a lower profile and sits nicely in the inferonasal quadrant.
• The patient has neovascular glaucoma. I use a valved implant in these patients because of some interesting data from the ABC Study that was presented a few years ago at the annual meeting of the American Glaucoma Society. (This data hasn’t been published yet.) In the ABC Study, a separate stratum was composed of patients with neovascular glaucoma; they were randomly assigned to either an Ahmed or Baerveldt implant. Remarkably, the data showed that neovascular glaucoma patients randomized to the Baerveldt group were about twice as likely to go blind as patients in the Ahmed group—a statistically significant difference.
What could explain a difference this large? I suspect it relates to ocular perfusion. Ocular perfusion is influenced by IOP and blood pressure—higher IOP and lower blood pressure are associated with lower ocular perfusion. Patients with neovascular glaucoma have impaired ocular perfusion, and this is the underlying cause of their disease. These patients typically have marked IOP elevation, and there appears to be a benefit of producing immediate pressure reduction with a valved implant, which results in improved ocular perfusion.
Beyond concerns such as these, choosing between the nonvalved implants is largely based on surgeons’ preference. The new ClearPath implant has a lot of design features that are similar to the Baerveldt, especially with respect to surface area, and we don’t have a lot of data that would suggest one is superior to the other. I’m mostly concerned with the surface area rather than the brand of implant, since the degree of pressure reduction seems to depend on that. (I have no financial interest in any of the implants.) If a nonvalved implant is my choice for a patient, I generally pick one that has a large-surface-area endplate, such as the 350 Baerveldt or 350 ClearPath, expecting that it will produce a greater IOP reduction.
Bridging the Gap
When implanting a nonvalved device like a Baerveldt or ClearPath, the surgeon must temporarily restrict flow through the device to avoid postoperative hypotony. The capsule surrounding the endplate provides the resistance to aqueous outflow, but that capsule takes several weeks to develop. I usually temporarily restrict flow through the tube by ligating it with a 7-0 vicryl suture. That suture will dissolve four to six weeks after surgery—adequate time to allow the capsule to form around the endplate.
However, this approach makes the tube shunt nonfunctional. There are several ways to provide IOP control in the early postoperative period until the tube opens:
• Restart medical therapy. One approach is to simply put the patient back on medical therapy. That probably wasn’t adequate to provide long-term pressure reduction or you wouldn’t be doing surgery, but it might be adequate as a temporizing measure until the tube opens up.
• Orphan trabeculectomy. Another option is doing an orphan trabeculectomy at the same time as your tube shunt surgery. Of course, you probably decided to implant a tube shunt because you felt a trabeculectomy would have a high probability of failure. However, an orphan trabeculectomy is one that you’re only counting on to work for a few weeks, to keep the pressure low until the tube opens up and provides more long-term pressure reduction.
• Create venting slits in the tube in front of the tie-off point. This is referred to as tube fenestration. When I make fenestrations, I usually make several, using a TG 140 needle (the spatulated needle on the vicryl suture). This approach is actually quite effective at providing pressure reduction, although it's a little less predictable than a trabeculectomy or using a valved implant.
• Create one slit in the tube and leave a suture in it to act as a wick. I learned this technique from Jamie Brandt, MD. He makes a little slit in the tube, like a standard fenestration, but leaves a 9-0 monofilament vicryl micro suture in the tube to serve as a wick, promoting aqueous egress. He calls this the “vent and stent” technique. It’s another option for early pressure reduction when using nonvalved implants.
Inferonasal Placement
We usually put tube shunts in the superotemporal quadrant, because the surgical exposure is generally easiest in that area. However, there are some reasons to do otherwise, and in those situations, Paul Sidoti, MD, has pointed out the value of inferonasal placement.
Reasons for avoiding superotemporal placement of a tube shunt include:
- There’s already an implant in the superotemporal quadrant, and a single tube shunt isn’t sufficient to control the pressure.
- There’s a lot of scarring of the conjunctiva superiorly.
- There’s a very large filtering bleb above that you’d like to avoid.
- The eye has thinning of the sclera. Patients with rheumatoid arthritis or a history of scleritis may have progressive thinning of the sclera.
- The cornea may be scarred superiorly, making it difficult to see the tube.
- There are a lot of peripheral anterior synechiae superiorly and you’re planning anterior chamber tube placement.
- The eye has silicone oil in it (sometimes used when treating complex retinal detachments). In this situation we generally don’t like to put a tube superiorly, because if any silicone oil migrates into the anterior chamber—which occasionally happens—it will find its way into the tube and drain into the subconjunctival space. In contrast, oil isn’t going to go towards an inferior location because it floats.
As it happens, putting an implant into the inferonasal quadrant is easy. In many ways it’s just as easy as putting it in the superotemporal quadrant. So, I have a pretty low threshold for putting an implant in the inferonasal quadrant. (You also can put valved tube shunts like the Ahmed in this quadrant.)
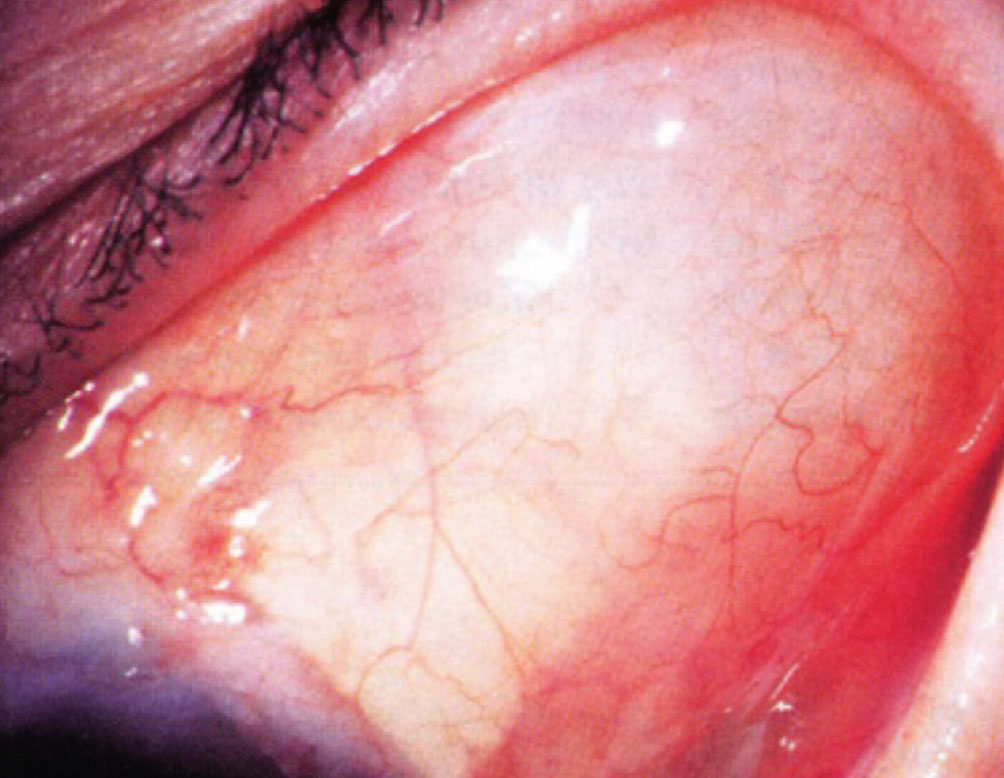 |
|
A postoperative photo showing a bleb overlying the endplate of a Baerveldt glaucoma implant. |
A few other tips when placing a tube in the inferonasal quadrant:
• Be careful in patients with small eyes, such as infants or patients with nanopthalmos. A small eye can be problematic because the optic nerve inserts into the back of the eye a bit nasally, making it possible that the endplate could press on the optic nerve. (Sharon Freedman, MD, at Duke University has a wealth of experience treating pediatric glaucoma; she’s developed an online calculator that will tell you how far back you can place the endplate to avoid encroaching on the optic nerve, as long as you know the patient’s axial length. It’s available to anyone at people.duke.edu/~freed003/GDDCalculator/. It’s a great resource in these unusual circumstances.)
• Route the tube to the 6 o’clock position. There’s less coverage of the scleral surface by the lower lid compared to the upper lid. Having inferior tubes routed to the 6 o’clock position maximizes the amount of tube that’s covered and protected by the eyelid.
• Use a corneal patch graft. With inferior tube placement, there’s less coverage by the eyelid, so I always use a transparent corneal graft. (These grafts are readily available.) You can use this for a superior placement as well, but in that situation it’s OK to use sclera, or other nontransparent materials, because the patch graft is usually fully covered by the upper lid.
Other Pearls
These strategies will also help ensure a good outcome:
• When creating a track for the tube, make sure you’re entering the anterior chamber parallel to the iris plane, and lift the needle a little bit as you enter the chamber. In my experience, creating a track for tube entry is the only part of the implantation procedure that requires a bit of finesse. One of the major complications from tube shunts is corneal decompensation. That’s largely related to the position of the tube—in particular, if it’s too close to the cornea.
The technique of lifting the needle upward towards the microscope a little bit as you’re entering the anterior chamber helps prevent the tube from ending up more anterior than you had planned.
• Make a longer tube track through the sclera. It’s important to minimize the risk of tube erosion after the surgery. Making a longer track by tunneling several millimeters in the sclera before ultimately entering the anterior chamber serves to minimize the risk of the tube eroding through the sclera and conjunctiva, which would increase the risk for endophthalmitis. (An erosion usually requires a trip back to the OR to resolve it.)
To further reduce the risk of tube erosion I also put a patch graft over the limbal portion of the tube. I think of this as a “belt-and-suspenders” approach to help prevent any chance of erosion.
• If you’re not happy with the position of the tube in the anterior chamber, take it out and make another entry incision right next to the first one, until you’re happy with the tube position. The tube is frequently not perfectly positioned on the first try. When that happens, I just make another entry incision. If I’m not happy with the tube position intraoperatively, it’s unlikely that I’ll be happy with it postoperatively. So, take your time and make sure you get it right while you’re in the OR.
• If you’re implanting into a child’s eye, leave a little extra tube length on the outside of the eye. This is another tip from Dr. Freedman. Route the tube in an “S” configuration on the scleral surface rather than making a direct entry path into the eye. Children’s eyes are still growing, so the tube could eventually retract from the eye if there isn’t adequate length.
• If a scleral buckle is present, position the implant based on the position of the buckle. If the buckle is very anterior in location, you can slip the implant behind it. But if the buckle is more posteriorly placed, you can position the endplate on top of the buckle. In this situation, I prefer to use a Baerveldt or ClearPath, because they both have a very low profile, which makes sense when your placing one implant on top of another.
• When placing an Ahmed implant, fill the anterior chamber with Healon at the end of the procedure. We want to avoid large drops in IOP in glaucoma patients, because that’s a known risk factor for suprachoroidal hemorrhage. However, in patients with very high preoperative pressure I often use valved implants like the Ahmed, where the tube is open from the beginning. This Healon strategy, which I learned from Kuldev Singh, MD, allows for a more gradual reduction of pressure as the viscoelastic slowly drains during the first couple of days following the procedure. I haven’t had any problems with postop pressure spikes using this approach.
Dr. Gedde is a professor of ophthalmology and vice chair of education at the Bascom Palmer Eye Institute in Miami. He has no personal financial ties to any product mentioned.