Optos has unveiled the 200Tx, its new- est ultra-widefield retinal imaging device. De- signed specifically for general ophthalmologists and vitreoretinal specialists, the device supports the diagnosis, analysis, documentation and monitoring of ocular pathology in the periphery of the retina.
The 200Tx is the first imaging device that provides visualization of ultra-widefield autofluorescence changes to retinal pigment epithelium. The 200Tx offers multiple wavelength imaging, including options for color, red-free, fluorescein angiography and autofluorescence, each of which can reveal different pathologies. The device also provides simultaneous pole-to-periphery views of more than 80 percent or 200 degrees of the retina in a single capture, helping physicians discover evidence of disease and guide treatment decisions.
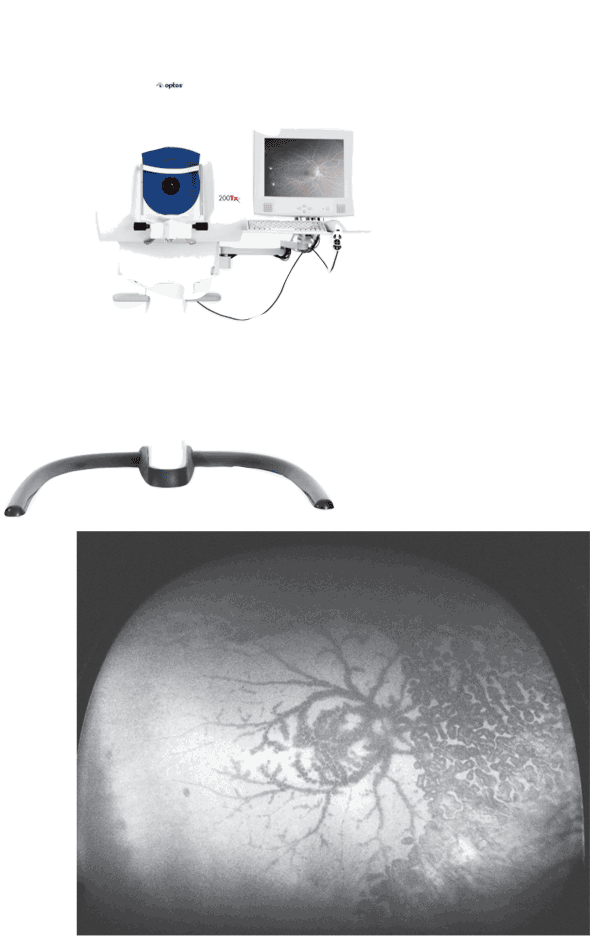
Srinivas Sadda, MD, of the Doheny Eye Institute,
The 200Tx will also be used to determine the frequency and significance of peripheral retinal abnormalities as part of the Age-Related Eye Disease Study 2, which is being conducted to determine methods of slowing the progression of vision loss from AMD.
The 200Tx device will be available for sale in early 2011. The device will be sold through a range of business models, from pay-per-patient rental agreements to outright capital sale. For information, visit optos.com.
Alcon
Alcon announced the
TobraDex ST
Normative Database Cleared for
Heidelberg Engineering an-nounced that the FDA has granted clearance for the new Spectralis age-adjusted RNFL thickness normative database. This FDA-cleared normative database gives clinicians a tool for assessing glaucoma risk from a patient's first visit. Combined with unique Spectralis features including FoDi fovea-to-disc alignment software and the new, Posterior Pole Asymmetry Analysis, the normative database increases the power of Spectralis for glaucoma risk assessment and progression management, the company says. In peer-reviewed journal articles, Spectralis has been shown to offer precise measurement reproducibly, which is important in glaucoma assessment.
The new normative database, Asym-metry Analysis, and other new features are a part of the Spectralis Version 5.3 software. The company plans to begin shipping the new software by mid-November. For information, visit heidelbergengineering.com.
TearLab Reports Reimbursement Recommended for Osmolarity Test
TearLab Corp. announced that the Centers for Medicare & Medicaid Services has published their recommended payment determination for new test codes, which includes a proposed reimbursement rate for the TearLab Osmolarity Test, effective January 2011. The new CPT code that will apply to the TearLab Osmolarity test will be reimbursed by CMS at $24.01 per eye. The actual CPT code will be published by the AMA in December 2010. Reimbursement by CMS will only be available for offices that have a Moderate Complex CLIA certificate until TearLab receives a CLIA Waiver categorization from the FDA. This waiver is currently under review by the FDA. The TearLab Osmolarity System uses a novel lab-on-a-chip approach that requires less than 50 nanoliters of tear fluid in order to measure tear osmolarity. The system eliminates the challenges that previously prevented point-of-care osmolarity testing, the company says, and can produce a sample-to-answer result in less than 30 seconds. For information, visit tearlab.com.
B+L Adds Tru-Size Diamond Knife, Relaunches PreserVision Vitamins
Bausch + Lomb Storz Ophthal-mic Instruments says its Tru-Size Dia-mond Knife enhances the already popular Storz profile diamond knife with incision widths engraved on the diamond blade, ensuring accuracy of the incision size. The profile design of the diamond blade features an asymmetric edge to ensure a straight tunnel through the cornea, allowing for easier creation of water-tight incisions while producing less corneal distortion.
A tip to shoulder length of 1.75 mm provides the surgeon with the extra diamond blade length often necessary in clear corneal incisions. This unique, marked diamond blade is now available in a 1.8-mm to 2.2-mm trapezoid knife design for microincision cataract surgery under product number E0130 1.8/2.2 For more information, to view a video of the instrument in surgery or to request a quote, visit the Storz Ophthalmic Instruments web site at storzeye.com.
B+L has also released a redesign of its PreserVision Eye Vitamin AREDS 2 Formula. The new, smaller soft gel, redesigned based on customer feedback, is dosed twice per day, two pills per dose. The product was voluntarily recalled in July 2010 due to concerns over the pill's original size.
PreserVision Eye Vitamin AREDS 2 Formula builds on the original PreserVision Eye Vitamin Age-Related Eye Disease Study Formula by adding omega-3 fatty acids (1,000 mg) per daily dosage, lutein (10 mg) and zeaxanthin (2 mg), replacing beta-carotene. Scientific studies show that the inclusion of high levels of omega-3 fatty acids, lutein and zeaxanthin in the diet supports eye health. For information, visit bausch.com.

Hand and Stand Magnifiers
Tech Optics International has introduced Task-Vision LED Hand and Stand Magnifiers available in powers 3X/12D , 4X/16D, 5X/20D, 7X/23D, 8X/28D, 10X/36D, 12X/44D and 14X/50D. They feature aspheric lenses with anti-reflective blue coating for superior optics; an easy-change battery lid; a large raised switch that's easy to use, especially for arthritic fingers; and a Flicker Free Cool long-lasting LED lighting with even illumination. They're available in round and rectangular. Call 1 (800) 678-0002, e-mail info@techopticsinternational.com or visit TechOpticsInternational.com.



