Epidemiology
Idiopathic intracranial hypertension was originally described in adults in 1893 and labeled “meningitis serosa,” but has also been called “pseudotumor cerebri” and “benign intracranial hypertension.”1 The incidence is approximately one in 100,000 individuals and can occur in all age groups, either gender and both obese and non-obese patients.2 Typically, however, the presenting patient is an overweight female of childbearing age. The most common symptoms include headache, binocular horizontal diplopia from bilateral sixth
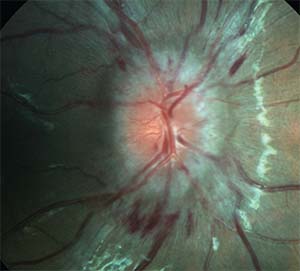 |
| Figure 1. Fundus photograph illustrating optic nerve edema with retinal hemorrhages in a 12-year-old boy with IIH. |
IIH occurs most commonly in young adults, and is rare in patients over age 45 or younger than age 3.4 There is no racial predisposition for the disease. No genetic cause has been described, although familial cases have been reported. Overall, the incidence of IIH in children is from 0.5 to 0.9 cases per 100,000. Among adults, overweight females have a strong predilection for the disease; in fact, the incidence rises to 19.3 per 100,000 in women who are 20 percent or more over their ideal body weight.5 Two-thirds of men and 90 percent of women with IIH are obese.4
In contrast, recent studies have shown that this disease doesn’t follow the same demographics in children as in adults. A study of pediatric patients with IIH recently demonstrated that only 43 percent of prepubertal children, compared to 81 percent of children aged 12 to 14 and 91 percent of teenagers aged 15 to 17, were obese.6 The same study also reported that only half of children with IIH under 12 years old were girls, while females comprised 88 percent of those IIH patients age 12 to 14, and 100 percent of those older than 14. The unequal sex distribution and the predilection for overweight patients seems to only begin after puberty, likely as a result of hormonal changes.7,8 Based on these findings, it appears that in the prepubescent population, the predilections towards obesity and female gender do not apply, and these risk factors may not be helpful clinical markers.
Pathophysiology
It is generally believed that the ultimate pathophysiology behind IIH leads to either increased cerebrospinal fluid production by the arachnoid villi or decreased absorption by the choroid plexus; however, despite many different theories, the precise pathway leading to IIH is unknown. Several studies support the hypothesis of increased cerebrospinal fluid production as the crucial pathophysiologic dysfunction in IIH. Studies have demonstrated decreased CSF protein concentration in patients in the adult and pediatric populations with IIH, suggesting increased CSF production.9,10 However, another study did not confirm this correlation between increased opening pressure and CSF protein concentration.11 Intracranial venous hypertension is a well-established finding in IIH, but it is unclear whether it is the cause or the result of increased ICP. Another hypothesis postulates that obesity leads to increased thoracic pressure, which causes an increase in both intracerebral venous pressure and ICP. A metabolic etiology has also been hypothesized, as numerous hormones, including cortisol, thyroid hormone, growth hormone and aldosterone are known to interact with receptors similar to transporters in the choroid plexus.12 Another mystery of IIH involves the ventricle. Despite the increase in ICP, ventricles in IIH do not increase in size.4
Vision loss in IIH is caused by compression of the optic nerve, as increased ICP is transmitted down the optic nerve sheath. Sixth cranial nerve palsies are the result of transmission of increased ICP to the abducens nerve as it travels through Dorello’s canal.
Secondary Causes
Vitamin A toxicity has long been known to cause IIH. This association was first reported amongst explorers consuming polar bear liver, which has very high levels of vitamin A.13 Medications containing vitamin A are commonly prescribed to adolescents for acne. Tetracycline antibiotics also commonly precipitate IIH.14 Other medications associated with IIH include:
• cyclosporine;
• cytarabine;
• nalidixic acid;
• lithium;
• minocycline;
• steroids and steroid withdrawal;
• danazol;
• nitrofurantoin;
• amiodarone;
• thyroid replacement therapy;
• human growth hormone;
• leuprorelin acetate;
• desmopressin;
• oral contraceptives; and
• all-trans retinoic acid.3,4
Certain medical conditions are also known to confer a higher risk of IIH. Several studies have illustrated a link between many types of anemia and IIH.3 Sickle-cell disease patients also have an increased risk of IIH.16,17 The prevalence of IIH in Down syndrome is 3.4 percent, which is much higher than that found in the general pediatric population.15 Turner syndrome also confers an increased risk for IIH, with or without the need for growth hormone.18,19
Clinical Presentation
The clinical presentation of pediatric IIH is similar to that in adults. Headache is the most common symptom and is present in approximately 90 percent of patients.20 There is no headache pattern that is specific for IIH. IIH may also present with no symptoms after the discovery of elevated or frankly edematous optic nerves on routine eye exam. Other
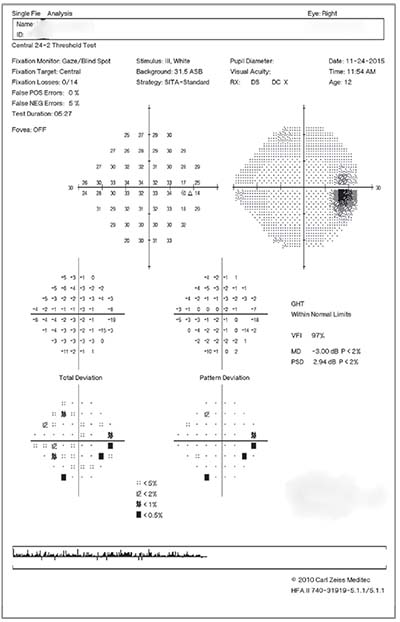 |
| Figure 2. Humphrey visual field showing enlargement of both blind spots in a child with papilledema from IIH. |
Workup & Diagnosis
A thorough history is the first step in a proper workup when IIH is suspected; it should focus on uncovering risk factors and symptoms consistent with increased ICP. The evaluating ophthalmologist should ask direct questions specifically regarding blurry vision, transient visual obscurations, binocular horizontal diplopia and pulsatile tinnitus. A complete list of medications should complement questions to probe for underlying genetic disease, endocrine conditions or a history of anemia. A nutritional history may also be of use, particularly in adolescents whose obesity may contribute to their condition.
The neuro-ophthalmic exam should include visual acuity, pupillary evaluation for a relative afferent defect, color vision, extraocular motility, slit lamp exam, dilated fundus exam and visual fields. Either automated visual fields or Goldmann visual fields are acceptable; however, many younger children will be unable to participate. For such patients, confrontation fields should be documented, if possible. Normative values for peripapillary nerve fiber layer thickness in children are currently unavailable; however, an OCT image should be obtained to document baseline thickness and evaluate improvement over time. If there’s any doubt about whether the patient has optic nerve edema, a B-scan ultrasound should be obtained looking for optic nerve head drusen, which is a common cause of misdiagnosis.
Neuroimaging to rule out an intracranial mass should be obtained before lumbar puncture to avoid the possibility of brain herniation. The study of choice is an MRI of the brain and orbits with and without contrast. Magnetic resonance venography should also be obtained in adults to rule out dural sinus thrombosis; this is a rare cause of increased ICP in children, however, and its utility may be limited for patients without an extenuating clinical circumstance. MRI findings associated with increased ICP include protrusion of the optic nerve head into the globe, enhancement of the optic nerve head, tortuosity of the intraorbital optic nerve, increased perioptic CSF, flattening of the posterior sclera, empty sella, cerebellar tonsillar descent, meningoceles and enlargement of Meckel’s cave.3
A complete blood count should be obtained to screen for anemia. After neuroimaging, a lumbar puncture should be performed with a measurement of opening pressure. The CSF constituents must be normal, excluding other causes of increased ICP, like meningitis. Opening pressure must be high to make the diagnosis of ICP, but this determination warrants special considerations with children. Recently, a study established a normal opening pressure of 19.6 cm H20 in children, with lower and upper limits of normal as 10.5 cm H20 and 28 cm H20, respectively.23 Recent updates to the modified Dandy criteria reflect these parameters.24 The study also reported that children who received significant sedation had a higher opening pressure by 3 cm H20 on average. Since many children must be sedated to perform a lumbar puncture, this finding should lead the clinician to interpret opening pressures with caution if the child has been sedated. The sedating physician should aim to provide the minimal amount of necessary anesthetic, and the overall clinical picture should be consistent with the opening pressure before beginning treatment.
Treatment
Before initiating treatment, medications that are known to precipitate IIH should be discontinued, if possible. In addition, if obese, the patient and parents should be counseled that weight loss of approximately 10 percent of body weight has been shown to provide significant benefit for patients with IIH.25 Medical treatments are generally aimed at decreasing CSF production by interfering with carbonic anhydrase, which is critical to its production by the choroid plexus. Acetazolamide is the most commonly used first-line agent, which has been shown to be superior to placebo and improve visual field defects from IIH.26 Common side effects include gastrointestinal upset, parasthesias, malaise, nephrolithiasis, and an altered taste for carbonated beverages.3 Topiramate is an anti-epileptic that also works to inhibit carbonic anhydrase and can be used as a primary or adjunct treatment for IIH. A recent study directly comparing acetazolamide to topiramate showed approximately equal efficacy.27 Topiramate also promotes weight loss, which may help obese adolescents.28 Furosemide can also be used as medical treatment for IIH, but may provide only a modest reduction in ICP.
When medical treatment fails, surgical treatment to reduce ICP must be considered. The most commonly performed procedure is placement of a ventriculoperitoneal or lumboperitoneal shunt. These procedures are able to achieve immediate reduction in ICP that may be necessary when visual field loss is severe.3 Optic nerve sheath fenestration is also a viable option for surgical management of IIH, though it’s not been shown to be either superior or inferior to shunt placement.29 A patient with severe headaches may benefit more from shunt placement, while patients who are otherwise poor surgical candidates may be good candidates for optic nerve sheath fenestration, which is less invasive. Recently, transverse venous sinus stenting in an attempt to increase venous outflow has been proposed as a treatment for IIH. This procedure has been reported in children, but long-term safety has not yet been established.30
Generally, the outcomes in pediatric IIH are favorable, with most children improved with only medical management. One study reported the visual outcomes of 68 children with IIH and determined that the majority of those who presented with either visual acuity or field defects had near complete recovery.31
Pediatric IIH in young children represents a unique entity and isn’t necessarily associated with female sex or obesity. Older children and adolescents with the disease tend to have demographic characteristics similar to adults. Normal parameters for ICP are different for children and opening pressures can be considered normal until above 28 cm H20. The treating clinician should be aware that sedation could significantly raise ICP and lead to a falsely positive high opening pressure. A mildly increased opening pressure should only be treated if it makes sense as part of the child’s complete clinical picture. Outcomes are generally positive, and the disease can be treated either medically or surgically. REVIEW
Dr. Fecarotta is in private practice.
1. Quincke H. Uber meningitis serosa and verewandte zustande. Deutsche Zeitschrift fur Nervenheilkunde 1897;9:149-168.
2. Durcan F, Corbett J, Wall M. The incidence of pseudotumor cerebri: Population studies in Iowa and Louisiana. Arch Neurol 1988;45:875-7.
3. Hartmann, AJ, Peragallo, JH. Pediatric idiopathic intracranial hypertension. J Pediatr Neurol DOI: 10.1055/s-0036-1593848.
4. Mercille G, Ospina LH. Pediatric idiopathic intracranial hypertension: A review. Ped Review 2007;28:e77-86.
5. Friedman DI. Pseudotumor cerebri. Neurol Clin North Am 2004;22:99-131.
6. Balcer LJ, Liu GT, Forman S, et al. Idiopathic intracranial hypertension: Relation of age and obesity in children. Neurology 1999;52:4:870-872.
7. Cinciripinni GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, outcome. Am J Ophthalmol 1999;127:178-82.
8. Phillips PH, Repka MX, Lambert SR. Pseudotumor cerebri in children. J AAPOS 1998;2:33-38.
9. Chandra V, Bellur SN, Anderson RJ. Low CSF protein concentration in idiopathic pseudotumor cerebri. Ann Neurol 1986;19:1:80-82.
10. Margeta MA, Buckley EG, El-Dairi MA. Low cerebrospinal fluid protein in prepubertal children with idiopathic intracranial hyptertension. J AAPOS 2015;19:2:135-139.
11. Johnston PK, Corbett JJ, Maxner CE. Cerebrospinal fluid protein and opening pressure in idiopathic intracranial hypertension (pseudotumor cerebri). Neurology 1991;41:7:1040-1042.
12. Sheldon CA, Kwon YJ, Liu GT, McCormack SE. An integrated mechanism of pediatric pseudotumor cerebri syndrome: Evidence of bioenergetic and hormonal regulation of cerebrospinal fluid dynamics. Pediatr Res 2015;77:2:282-289.
13. Rodahl K, Moore T. The vitamin A content and toxicity of polar bear and seal liver. Biochem J 1943;37:2:166-168.
14. Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis 1995;55:3:165-168.
15. Esmaili N, Bradfield YS. Pseudotumor cerebri in children with Down syndrome. Ophthalmology 2007;114:9:1773-1778.
16. Segal S, Discepola M. Idiopathic intracranial hypertension and sickle cell disease: Two case reports. Can J Ophthalmol 2005;40:6:764-767.
17. Henry M, Driscoll MC, Miller M, Chang T, Miniti CP. Pseudotumor cerebri in children with sickle cell disease: a case series. Pediatrics 2004;113:e265-e269.
18. Bala P, McKiernan J, Gardiner C, O’Connor G, Murray A. Turner syndrome and benign intracranial hypertension with or without growth hormone treatment. J Pediatr Endocrinol Metab 2004;17:9:1243-1244.
19. Sybert VP, Bird TD, Salk DJ. Pseudotumor cerebri and the Turner syndrome. J Neurol Neurosurg Psychiatry 1985;48:2:164-166.
20. Wall M. The headache profile of idiopathic intracranial hypertension. Cephalgia 1990;10:331-5.
21. Rogers DL. A review of pediatric intracranial hypertension. Pediatric clinics. June 2014;61:3:579-590.
22. Schirmer CM, Hedges II TR. Mechanisms of visual loss in papilledema. Neurosurg Focus 2007;23:5:e5.
23. Avery, RA Shah SS, Licht DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med 2010;363:9:891-893.
24. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81:13:1159-1165.
25. Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: The association between weight loss and the requirement for systemic treatment. BMC Ophthalmol 2007;21:7:15.
26. Wall M, Johnson CA, Cello KE, Zamba KD, McDermott MP, Keltner JL; NORDIC Idiopathic Intracranial Hypertension Study Group. Visual field outcomes for the idiopathic intracranial hypertension treatment trial (IIHTT). Invest Ophthalmol Vis Sci 2016;57:3:805-812.
27. Celebisoy N, Gokcay F, Sirin H, Akyurekli O. Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open label study. Acta Neurol Scand 2007;116:5:322-327.
28. Saunders KH, Kumar RB, Igel LI, Aronne LJ. Pharmacologic approaches to weight management: Recent gains and shortfalls in combating obesity. Curr Atheroscler Rep 2016;18:7:36.
29. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumor cerebri: Optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol 2014;98:10:1360-1363.
30. Tibussek D, Distelmaier F, von Kries R, Mayatepek E. Pseudotumor cerebri in childhood and adolescence-results of a Germany wide ESPED survey. Klin Padiatr 2013;225:2:81-85.
31. Ravid S, Shahar E, Schif A, Yehudian S. Visual outcome and recurrence rate in children with idiopathic intracranial hypertension. J Child Neurol 2015;30:11:1448-1452.



