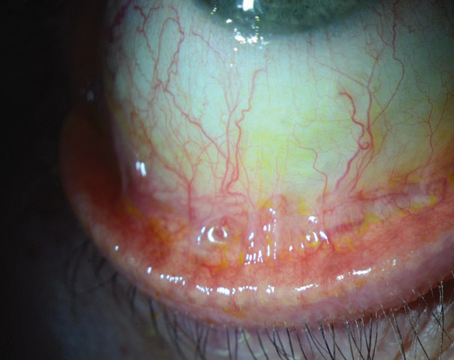
 |
| Tapping technique to unscroll a DMEK graft. |
Why DMEK?
DSAEK currently sits in the sweet spot in which it can provide good outcomes for patients with Fuchs’ and other posterior corneal dystrophies, as well as aphakic and pseudophakic bullous keratopathy, while, at the same time, not putting a lot of technical demands on the surgeon.
There are reasons to consider DMEK, however, as it can potentially offer even more benefits, such as:
• true anatomic replacement of Descemet’s membrane and endothelium, a 15 to 20-µm thick structure;
• pachymetric uniformity across the entire graft, which DSAEK can’t always achieve;
• lower antigenic stimulus—and therefore lower incidence of allograft rejection—by virtue of less tissue being transplanted;
• the need for less steroids to prevent immune rejection;
• a lower incidence of steroid-induced intraocular pressure elevation and glaucoma; and
• if done correctly, the possibility of quicker visual rehabilitation.1
DMEK, for all its advantages, isn’t for every patient, however. The ideal patient is a pseudophake with a posterior chamber lens implant, ideally one that’s situated in the capsular bag, with or without a posterior capsulotomy. For patients with serious co-morbidities, I still prefer to perform DSAEK. With this in mind, patient presentations in which to avoid DMEK—at least for the novice DMEK surgeon—are patients with:
• anterior chamber intraocular lens implants;
• unicameral eyes;
• vitreous prolapse into the anterior chamber;
• a history of trabeculectomy;
• a history of tube shunt implantation;2
• failed penetrating grafts; and
• failed previous DSAEK grafts.
The common themes among these relative contraindications include the possibility of losing the graft into the vitreous cavity, the difficulty you’ll have in unscrolling the graft in the eye and the potential increased challenge of attaching the graft.
The Four Challenges of DMEK
For surgeons experienced with DMEK, as well as surgeons just starting out, there are four areas that will test their skills. It’s these areas that currently hold DMEK back in terms of being a widely accepted technique. You can clear these hurdles, however. Here’s a look at why these stages are particularly challenging, and ways you can make them more manageable.
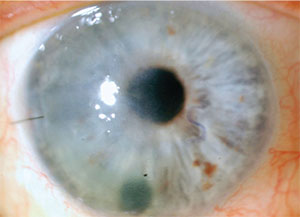 |
| Temporal corneal edema after DMEK secondary to a DMEK detachment. Note the letter S nasally in its correct orientation. |
• Preparation of the donor tissue. This challenge is not so much the actual pre-stripping of the tissue—the vast majority of us have trained an eye-bank technician to strip the tissue for us, and most of them have become better than us at this task. Instead, it’s more about an awareness of the donor’s age and diabetic status, and how both will affect the tissue’s behavior.
Corneal specialists have unofficially agreed that the tissue donor shouldn’t be younger than 50 years of age, diabetic or have had previous cataract surgery. Why is this? Because the younger the tissue, the harder it is to strip the Descemet’s membrane/endothelial complex off the donor cornea, and the tighter its subsequent scroll is when you try to unscroll it in the recipient’s anterior chamber. I won’t hesitate to accept a donor that’s 70 to 72 years of age, however, because the Cornea Donor Study showed there wasn’t much of a difference in risk of graft failure in grafts from donors up to age 75;3 I know I’ll be able to handle an older donor’s tissue inside the eye more easily. Secondly, diabetic patients’ tissue has been shown to be more difficult to strip and more likely to develop tears.
Finally, if the donor has had previous intraocular surgery, particularly cataract surgery, and especially if the cataract surgeon made a long phaco incision tunnel, you or the eye-bank tech may encounter the internal lumen of the old cataract incision while stripping the tissue. If this occurs, that’s a likely location for a tear.
Also, preparing the donor graft from perfectly pre-stripped tissue can be challenging, as punching, isolating and placing the graft into the delivery device is not without potential complications.
• Insertion of tissue into the anterior chamber. DMEK surgeons usually use one of two ways to insert the tissue: 1) a push-through technique by way of a glass tube or a modified intraocular lens inserter; or 2) a pull-through technique after placing the donor graft on a Busin glide, contact lens or similar platform. Most DMEK surgeons are currently using a push-through method. With this being the case, it’s important to note that the glass tube and modified IOL inserter have advantages and limitations.
The glass tube is actually a modified version of the Jones tube we’ve used for years for dacryocystorhinostomy procedures. The tube’s shape has been slightly modified by Michael Straiko, MD, of the Devers Eye Institute in Portland, Ore., creating what’s known as the Straiko-modified Jones tube. It can be inserted through a 3.2- to 3.5-mm incision. Proponents of these tubes believe that they do less endothelial cell damage if the graft tissue touches the glass wall of the tube, compared to the plastic interior of a modified IOL cartridge.
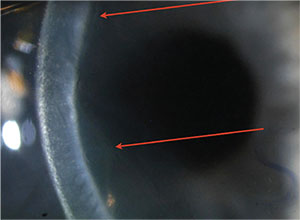 |
| Slit view of the detached DMEK graft from p. 26, highlighted by the arrows. |
One of the pearls for working with a glass tube is that it’s important to get a feel for how to aspirate the tissue into the tube. This is because you’re aspirating the tissue from a petri dish rather than placing it into the back of the tube. To get a feel for this, use peripheral fragments of Descemet’s membrane from the donor tissue after the punch, not the graft itself, to see how much to pull on the plunger to aspirate the tissue up into the tube.
Surgeons who use plastic, modified IOL injection cartridges argue that one of the main benefits of their approach is that the tissue can be inserted through a 2.4- to 2.8-mm incision. The main challenge with IOL inserters is that they’re not meant to push such thin tissue—about 20 µm thick—into the eye. This can cause issues as you work with the inserter (indeed, not all inserters are created equal for this purpose). This is because in the course of its normal operation, the inserter’s plunger doesn’t completely seal the channel that it goes into, and it doesn’t need to for the purpose of injecting an IOL. Because of this, though, saline solution can occasionally leak from the back of the cartridge—taking the thin DMEK graft with it and incarcerating it between the plunger and the wall of the cartridge. Note that this is more likely to occur if you use a chamber maintainer with fluid infusion during the insertion.
If you use either of these methods, glass tube or modified inserter, the number-one technique tip is to size the wound in such a way that you can insert the tube or cartridge into the incision tightly but without difficulty. You don’t want to encounter a problem with going through the wound after you’ve floated the graft into the glass tube or cartridge. Also, choose an inserter that allows for a tight seal between the plunger and the wall of the cartridge.
As mentioned above, there are ways to introduce the graft into the eye other than as a scroll, such as a method that involves placing the graft on a Busin glide or other platform, similar to the way in which the surgeon inserts a DSAEK graft, using a two-handed, pull-through technique. However, since most surgeons insert the DMEK graft into the eye as a scroll, that is what this article focuses on.
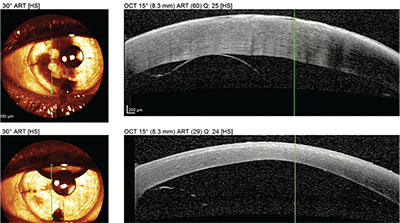 |
| The OCT appearance of the detached graft in the slit image above (top) and the OCT of the eye following reattachment with a rebubbling procedure (bottom). |
This brings us to the challenge of getting the graft oriented correctly when it unscrolls.
• Unscrolling the tissue and achieving successful tamponade. Getting the thin DMEK graft to unscroll can be a challenge, especially when the tissue is tightly scrolled.4 Several techniques have been developed to help you unscroll it, however.
First off, one way to achieve the correct orientation of the tissue as it transitions from a scroll to an unscrolled sheet is the marking method. The method involves the eye bank, or whoever prepares the tissue, marking the stromal side with a letter S to aid in the subsequent placement in the eye. An ideal way to make this mark is by using a stamper colored with a gentian violet surgical pen and allowing the alcohol to evaporate.5
Alternatively, you can intraoperatively discern the graft orientation once it’s in the eye using either a slit lamp attached to your operating microscope, or a tiny handheld slit lamp. As the graft unscrolls it will begin to take on the shape of the letter C, with the endothelium on the outside of the C. So, if by using the slit beam you can tell the orientation of the C, you’ll always know which side is up; you want the inside of the C to be facing up, so it looks like a cup under the dome of the cornea.
Aside from these popular approaches, there are other published techniques to aid in determining the orientation of the graft, too. Of course, an operating microscope with built-in OCT will accomplish that goal … at a significantly higher cost.
In terms of getting the graft to unscroll, one method used by many surgeons is the tapping technique, developed by Efdal Yoeruek, MD, of Eberhard-Karls University in
Tübingen, Germany. The technique involves bouncing an instrument, usually a BSS or 27- or 30-ga cannula, on the cornea over the location of the scroll together with a simultaneously shallowing of the anterior chamber. This tapping sends fluid waves through the anterior chamber that unscroll the tissue. This technique usually works best if the chamber is shallow enough so that, when you start unscrolling the graft, it doesn’t just scroll right back again. A good rule of thumb for this chamber shallowing is to make it less than one corneal thickness deep. For example, if the average cornea is 500 to 600 µm thick, the anterior chamber should be roughly 0.5 mm or less in depth after the graft is unscrolled, as opposed to its usual 2.5 to 3.5 mm depth, in order to successfully tap and unscroll the tissue and keep it unscrolled until the air/gas bubble is injected.
Selective irrigation is another method that can help unscroll the tissue. To understand this, it helps to know that most DMEK surgeons will make two to four paracenteses that are used for many functions during the procedure: One use for the paracenteses is to deepen the chamber when necessary. For example, if, after you unscroll the tissue you find that it’s upside down—i.e., you notice the letter S marking is inverted—you need to deepen the anterior chamber to release the tissue and scroll it again so you can flip it over. Another use for the paracenteses is to use the irrigation to move the tissue toward the center of the chamber. A third use of irrigation is if the tissue is in a double-scroll configuration, or even a three-corner folded graft (think Napoleon’s hat), the fluid pulses can be used to open up the tissue. My colleague in India, Rajesh Fogla, MD, has developed a cannula with multiple irrigation holes dubbed the Fogla DMEK Cannula (Storz Ophthalmics) that is situated inside the scroll. Once the Fogla cannula is inside the scroll, the surgeon injects the fluid and the scroll opens.
Another method that some may consider dexterity-intensive, but which is exciting to watch, is called the bubble roll, developed by Friedrich Kruse, MD, of Erlangen, Germany.6 With this technique, when the graft is inside the injector cartridge, the surgeon places a small bubble inside the scroll. Then, when the graft enters the eye, he can place pressure on the dome of the cornea to move the bubble around inside the scroll to unscroll it.
For my own part, I’ve teamed with Phillipsburg, N.J., ophthalmologist, William B. Neusidl to develop an instrument for unscrolling the DMEK graft. We’ve dubbed it the Neusidl-Hannush Loop, or NHL. The NHL’s operation involves a polypropylene thread inside a metal lumen that’s pulled taut and inserted into the anterior chamber and inside the DMEK scroll. An actuator then opens the loop to unscroll the graft. The instrument has been tested successfully in the wet lab, and is currently in the manufacturing process.
Once the graft is unscrolled, the surgeon has to tamponade it to the host cornea. For this, the options are to use a bubble composed of either air or 20% SF6. The main advantage of the latter is that it lasts longer, which may be helpful in achieving full apposition of the DMEK graft to the host cornea. With DSAEK you mainly need to tamponade the graft for 10 minutes with a pressure above 30 mmHG and the graft will usually stay in place, as long as there’s no fluid between the graft and the host. With DMEK things are different. For DMEK, many surgeons feel that it’s important to have a longer-lasting bubble and to make sure the patient lies on his/her back postop for at least the first 24 hours, then at least a few hours a day for the next several days. So for DMEK a longer-lasting SF6 bubble can make a difference; e.g., an 80-percent air bubble may last three to four days, while an 80-percent SF6 bubble can last twice as long, and might help increase your chance of success with DMEK graft attachment. Also, remember all DMEK patients should receive an inferior iridotomy. I should also mention here that a plethora of online videos can be of great help to the novice DMEK surgeon, both in facilitating the successful completion of the procedure and in avoiding and managing complications.
• Postop management. When DMEK surgeons discuss postop management, we’re usually referring to decreasing the incidence of and managing graft detachment. To decrease this risk, it’s important that the patient lie on his back, take the drops, refrain from rubbing the eye and keep up with the follow-up visits.
In terms of how long the patient must remain on his back, if we’ve used SF6, which can last about a week, we ask the patient to be on his back continuously for the first day, and then for two hours in the morning and two hours in the afternoon for the next five days postop. Since I frequently remove the central epithelium at the time of surgery, a bandage contact lens is used for the first week. It must be noted, however, that not every surgeon performs the keratectomy/contact lens step.
In addition to a postop steroid and antibiotic, my postop medical regimen usually includes Muro 128 hypertonic solution to promote deturgescence of the host stroma. I usually start the Muro the second week after the surface has healed and the antibiotic has been discontinued.
 |
| The eye from pg. 29, with the graft attached after a rebubbling procedure. |
The main concern during the postop course is how to respond if the graft doesn’t adhere completely. In my practice, the incidence of partial graft non-attachment requiring rebubbling (complete detachment is very rare) is 20 percent, vs. less than 5 percent for DSAEK. Studies have also found a higher rebubbling rate with DMEK.7 Netherlands surgeon and DMEK originator Gerritt Melles, MD, has argued that if less than one-third of the graft isn’t adhered, especially if the area of non-attachment is not visually significant, the surgeon may observe the patient, since grafts that are 60- to 70-percent attached will almost always completely attach and stay in place in time. That’s also been my experience for the most part. However, I also think that the longer you leave the graft unattached, the worse the visual prognosis becomes, due to chronic edema. Therefore, if the area of detachment extends over the undilated pupillary aperture, I prefer to rebubble—create a new bubble for tamponade—usually between 10 and 20 days postop, a strategy I’ve learned from Dr. Kruse and that has served my patients well. If I find it challenging to discern the existence or extent of the detachment, especially if it’s very shallow and in the setting of corneal edema, anterior segment OCT has been very helpful in determining the presence of a graft detachment and its extent.
In practice, if I see a DMEK patient a week postop and notice that the cornea is a little edematous, and I can’t see the graft well—and then perform an OCT and find a shallow detachment—I’ll inform the patient that I’ll most likely want to put another bubble in the eye the following week. In preparation for this appointment, I advise the patient to bring someone along for assistance, and to be prepared to spend a couple of hours at my office, which is where I perform almost all rebubbling procedures. Some surgeons prefer to do it in the OR, and still others do it in a minor procedure room.
At that rebubbling visit, I sit the patient at the slit lamp and use one of the existing paracenteses to inject air for the creation of the new bubble. A note for rebubbling: With DSAEK, you only need to insert the air cannula a little bit into the anterior chamber and, as long as you’re behind the graft, you can inject the air. With DMEK, however, if you’re too peripheral when the bubble starts to form, you can push the graft aside like an accordion and detach it completely. Therefore, make sure the cannula is in the center of the anterior chamber behind the graft before you start injecting the air.
Though rebubbling is an effective response to graft non-attachment, it’s important to note that graft failure can still occur for a multitude of reasons. Graft failure may occur due to poor attachment, an upside-down graft or rejection. In my hands, and I believe those of many colleagues, graft failure may be a function of marked manipulation of the graft. This manipulation usually occurs intraoperatively during the unscrolling step, in what is referred to as “the dance,” and leads to a significant loss of endothelium. In the literature, the rate of primary graft failure with DMEK varies from 2.5 to 10 percent.8-10
Techniques in Development
Though DSAEK may be entrenched as the less-invasive alternative to penetrating keratoplasty for the indication of endothelial dysfunction, as well as the one associated with better visual results than PK (mainly because of reduced astigmatism), and DMEK is the more technically challenging—but potentially better—aspirant to the throne, there are other approaches to endothelial keratoplasty that are worth noting.
• Ultra-thin DSAEK. In an effort to achieve some of the benefits of DMEK but perhaps with an easier time working with the graft, Italy’s Massimo Busin, MD, and co-workers developed UT-DSAEK.11 In practice, UT-DSAEK involves preparing a DSAEK graft thinner than 100 µm by a combination of several methods: removing the epithelium before making the microkeratome pass; making a double-pass with the microkeratome; using a 300, 350, or even 400 µm microkeratome head; increasing the pressure in the anterior chamber to about 90 mmHG as you make the pass; and/or making a slower microkeratome pass.
Working with a thinner DSAEK graft is technically less challenging than a DMEK graft. Theoretically, it may offer better visual results than conventional DSAEK, mostly due to the increased uniformity and thickness of the donor tissue. There have been no formal studies showing the benefit of UT-DSAEK over DSAEK or DMEK, so the jury is currently still out on this. Anecdotally, however, many surgeons, myself included, seem to think patients do get better vision from a thinner graft. Therefore, I aim for that type of graft whenever possible.
• Redistributed endothelial cells. In the past several years, some ophthalmologists have questioned whether we need to perform a keratoplasty of any sort in cases of Fuchs’ endothelial dystrophy. Instead, they say, there may be a way to just coax the endothelium to return to its normal level of function, or to redistribute healthy endothelium to a central location where it may perform its function better.
The Netherlands’ Dr. Melles reported on an 80-year-old patient who underwent DMEK for Fuchs’, but had a detached graft hours after the procedure.12 The patient elected to wait and observe the situation because the eye had low visual potential. One month postoperatively, the cornea began to clear. At six months, only minor edema remained in the superior far periphery. The corneal pachymetry returned to normal and the endothelial cell density was 830 cells/mm2. After this result, Dr. Melles saw a potential therapy and tentatively called the practice of a descemetorhexis followed by the deposition of a free-floating Descemet’s scroll in the anterior chamber “Descemet’s membrane endothelial transfer,”or DMET. Dr. Melles found a similar result in a small group of Fuchs’ patients undergoing DMET, but not a group with bullous keratopathy.13
The question, of course, is whether the source of new endothelium centrally was the donor graft or the relatively healthy peripheral host endothelium in Fuchs’. The University of Chicago’s Kathryn Colby, MD, PhD, has also been working with a less-invasive way to treat some Fuchs’ patients. In her retrospective study, she stripped the central Descemet’s membrane in 13 eyes of 11 patients at the time of their cataract surgery. At a follow-up of six months and later (range: six to 24 months), 10 of the patients had corneal clearing, with a visible central endothelial cell mosaic, and saw between 20/20 and 20/15 (preop: 20/25 to 20/400).14
Though this approach seems to confirm what many of us suspected—that stripping the central 5 mm of endothelium in Fuchs’ dystrophy might allow the cornea to redistribute the remaining healthy endothelial cells from the periphery—we’re not sure if this approach works enough of the time to justify its widespread use. We’ll observe developments in this area with interest.
• Pre-Descemet’s Endothelial Keratoplasty. With PDEK, a procedure developed by India’s Amar Agarwal, MD, and Great Britain’s Harminder Dua, MD, the idea is to include the newly discovered (though disputed by some) Dua’s layer of the cornea in the posterior graft of endothelium and Descemet’s membrane.15 This makes the graft 30 to 40 µm thick, which is only slightly thicker than a DMEK graft but much easier to work with in several ways: 1) Since you’re not stripping Descemet’s from stroma, the risk of damaging Descemet’s membrane is eliminated. 2) You’re not restricted to using donors older than 50 because you don’t need to worry about tearing Descemet’s membrane during stripping as can occur in DMEK. 3) A PDEK scroll is 30 to 40 µm thick, not 15 or 20 µm as in DMEK, so it’s easier to unscroll in the anterior chamber.
The challenge, however, is in harvesting the tissue for PDEK, which is the main reason the technique hasn’t caught on yet. To harvest the donor graft, you can’t strip it through a posterior approach. Instead you have to create a “big bubble” similar to that used in deep anterior lamellar keratoplasty. The eye-bank technician or the surgeon has to create this bubble so that, when it expands and separates the tissue, it yields a posterior layer that has all three layers needed for PDEK before trephining the graft. The challenge is that we don’t always achieve a big bubble, and, if we don’t achieve it, the corneal tissue is potentially permanently damaged and unusable.
• Rho-associated kinase inhibitor drops. Japan’s Shigeru Kinoshita, MD, has been developing a technique for the treatment of endothelial dysfunction that involves instilling ROCK inhibitor eye drops to promote endothelial healing. After finding accelerated healing due to the drops in animal models, he instilled them in eight patients with endothelial dysfunction and reported a positive effect.16 The next step in this research is ex-vivo expansion of healthy host endothelial cells.17
Though it’s true that DMEK is more challenging to perform than DSAEK, not many things in life or in ophthalmology that are worth doing are ever easy. If you can stay with DMEK through the learning curve and put to use some of these principles, you and your patients will often be rewarded with excellent outcomes. REVIEW
Dr. Hannush is an attending surgeon in the Cornea Service at Wills Eye Hospital, Department of Ophthalmology, Sidney Kimmel Medical College of Thomas Jefferson University. He is also the medical director of the Lions Eye Bank of Delaware Valley.
1. Price MO, Giebel AW, Fairchild KM, Price FW Jr. Descemet’s membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009;116:12:2361-8.
2. Ni N, Sperling B, Dai Y, Hannush S. Outcomes after Descemet stripping automated endothelial keratoplasty in patients with glaucoma drainage devices. Cornea 2015;34:8:870–875.
3. Cornea Donor Study Investigator Group, Gal RL, Dontchev M, Beck RW, et al. The effect of donor age on corneal transplantation outcome results of the cornea donor study. Ophthalmology 2008;115:4:620-626.
4. Kruse FE, Laaser K, Cursiefen C, et al. A stepwise approach to donor preparation and insertion increases safety and outcome of Descemet membrane endothelial keratoplasty. Cornea 2011;30:5:580-587
5. Veldman PB, Dye PK, Holiman JD, Mayko ZM, Sáles CS, Straiko MD, Galloway JD, Terry MA. The s-stamp in Descemet membrane endothelial keratoplasty safely eliminates upside-down graft implantation. Ophthalmology 2016;123:1:161-4.
6. Kruse FE, Schrehardt US, Tourtas T. Optimizing outcomes with Descemet’s membrane endothelial keratoplasty. Curr Opin Ophthalmol 2014;25:4:325-34.
7. Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: Prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology 2011;118:12:2368-73.
8. Hamzaoglu EC, Straiko MD, Mayko ZM, Sáles CS, Terry MA. The first 100 eyes of standardized Descemet stripping automated endothelial keratoplasty versus standardized Descemet membrane endothelial keratoplasty. Ophthalmology 2015;122:11:2193-9.
9. Deng SX, Sanchez PJ, Chen L. Clinical outcomes of Descemet membrane endothelial keratoplasty using eye bank-prepared tissues. Am J Ophthalmol 2015;159:3:590-6.
10. Terry MA, Straiko MD, Veldman PB, et al. Standardized DMEK technique: Reducing complications using prestripped tissue, novel glass injector, and sulfur hexafluoride (sf6) gas. Cornea 2015;34:8:845-52.
11. Busin M, Albe E. Does thickness matter: Ultrathin Descemet stripping automated endothelial keratoplasty. Curr Opin Ophthalmol 2014;25:4:312-8.
12. Dirisamer M, Ham L, Dapena I, van Dijk K, Melles G. Descemet membrane endothelial transfer: “Free-floating” donor Descemet’s implantation as a potential alternative to “keratoplasty.” Cornea 2012;31:2:194.
13. Dirisamer M, Yeh R, van Dijk K, Ham L, Dapena I, Melles G. Recipient endothelium may relate to corneal clearance in Descemet membrane endothelial transfer. Am J Ophthalmol 2012;154:2:290.
14. Borkar DS, Veldman P, Colby KA. Treatment of Fuchs’ endothelial dystrophy by descemet stripping without endothelial keratoplasty. Cornea 2016 Jun 15. [Epub ahead of print]
15. Altaan S, Gupta A, Sidney L, Elalfy M, Agarwal A, Dua H. Endothelial cell loss following tissue harvesting by pneumodissection for endothelial keratoplasty: An ex vivo study. Br J Ophthalmol 2015;99:5:710-713.
16. Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea 2014;33:11.S25-31.
17. Navaratnam J, Tor P. Utheim, et al. Substrates for expansion of corneal endothelial cells towards bioengineering of human corneal endothelium. J Funct Biomater 2015;6:3:917–945.