Now, a new point-of-care screening device, currently undergoing clinical trials, may be able to quickly eliminate or confirm common causes of conjunctivitis, making treatment much easier and more effective.
|
|
How It All Started
Rapid Pathogen Screening Inc. (RPS) is a joint venture between three ophthalmologists practicing in Binghamton, N.Y., (José Sambursky, MD and his two sons, Daniel Sambursky, MD, and Robert Sambursky, MD) and Securetec Detektions-Systeme AG, located in Munich, Germany. The Samburskys are clinical ophthalmologists with expertise in the management of infectious diseases; Securetec develops, manufactures and sells point-of-care biosensors, used by customs agents and police around the world to identify illegal narcotics, explosives and other (bio-)chemical agents.
Realizing that this technology could be a powerful tool for medical diagnosis, the Samburskys set up a cooperative research agreement with Securetec to explore the feasibility of POC detection and identification of infectious diseases. In March 2004, promising results led the Samburskys and Securetec to form RPS Inc. as a joint venture.
The new company's purpose is to develop, manufacture and market easy-to-use, point-of-care disposable diagnostic detectors that can confirm the presence of specific pathogens within 10 minutes. RPS plans to market them to ophthalmologists, optometrists and primary medical care practitioners.
Choosing Targets
RPS's Medical Director Robert Sambursky, MD, explains that the contagious nature of conjunctivitis made it seem like a good first disease choice, and of the multiple possible causes to detect, three stood out. "Adenovirus is often misdiagnosed as infection, and herpes and Chlamydia are difficult to diagnose," he notes.
The company's first product is a lateral flow immunoassay device designed to detect the presence of adenovirus in ocular fluids. Dr. Sambursky points out that adenovirus is the most frequent overall cause of conjunctivitis, and accounts for 60 to 90 percent of all viral cases. "We did a small study involving about 50 patients in the Wills Eye emergency room last summer," he says. "We found that conjunctivitis was caused by adenovirus about 60 percent of the time in the summer, although the number drops to 40 or 45 percent in the winter." (The Wills Eye conjunctivitis study has been submitted for publication.)"Also," he adds, "adenovirus is resistant to alcohol cleaning, so it can live on surfaces for 49 days."
Dr. Sambursky says studies have found that 90 percent of people who visit a primary-care doctor for conjunctivitis are put on an antibiotic. "Given the percentages of adenovirus our study found, probably half of the 90 percent who are being treated with antibiotics don't need them. As we all know, unnecessary antibiotics may contribute to bacterial resistance, and not all antibiotics are completely benign; there are problems with toxicity and allergy. Plus, antibiotics, especially the non-generic fluoroquinolones, are a significant factor in health-care costs."
Next: Herpes and Chlamydia
Dr. Sambursky says that RPS is also working on tests for Chlamydia and the herpes simplex virus, which are less frequent causes of conjunctivitis but have more devastating complications. "Within six to nine months we hope to have a triple test that will screen for all three pathogens simultaneously," he says.
Dr. Sambursky notes that there are many types of bacteria that can cause conjunctivitis beside Chlamydia, and other viruses as well, but he says the other viruses are only responsible for 10 to 15 percent of conjunctivitis cases. "At the moment," he adds, "we see more of a need for an allergy test than for additional infectious conjunctivitis parameters."
Dr. Sambursky can't comment on the precise accuracy or sensitivity of the test because it's still in clinical trials. However, he says the test is achieving sensitivities and specificities that parallel culturing the sample. And, he notes that using the test will save 60 to 90 percent of the cost of a standard laboratory test such as culture or polymerase chain reaction. (The RPS conjunctivitis detector has a shelf life of 24 months and can be stored at room temperature.)
The RPS Conjunctivitis Detector consists of a lateral flow immunoassay in a plastic housing, and a sterile sample collector that also functions as a cover for the housing.
"Using the blue collector, you take a fluid sample from the lower conjunctiva," explains Dr. Sambursky. "Then you connect it directly to the white plastic cassette. As the strip absorbs the sample the fluid is drawn across antibodies, revealing the presence or absence of the antigen. You never handle the strips directly, so there's no chance for contamination." Dr. Sambursky points out that RPS detectors are very sensitive because they don't dilute the sample.
"When someone comes in with potential conjunctivitis, you run the test on him," he says. "If it's positive for adenovirus, you prescribe supportive care. If the test is negative, there's still a chance that the cause could be a virus, but you have a higher probability that the culprit is a bacteria. You're more likely to have a better treatment outcome with an antibiotic and less likely to be overprescribing."
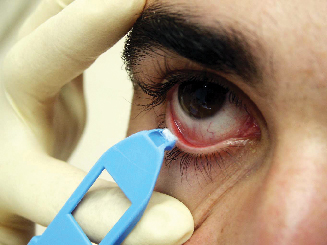 |
| The sample collector also functions as a cover for the housing. |
Using the Test In Practice
Shachar Tauber, MD, director of ophthalmology research and cornea and refractive surgery at St. John's Hospital and Clinics in Springfield, Mo., has been participating in clinical trials of the adenovirus detector. "Once this test is validated, the best place for it will be in the primary-care doctor's office," he says. "The primary-care doctor will have much more confidence that he or she isn't prescribing an antibiotic than may lead to resistance if used inappropriately. So ophthalmologists will get a lot fewer referrals for a problem we can't do much about, one that potentially puts our practices at risk."
Dr. Tauber says using the test is no different from taking a culture. "It doesn't hurt," he notes, "and you get an answer right away, not three to five days later. You just keep these stockpiled in a box and order them when you run out."
Dr. Tauber sees a number of additional advantages to using the adenovirus detector. "Patients demand treatment," he says, "and most doctors give the patient a prescription, because if they don't, the patient gets angry. Of course, then the patient is angry because the drop doesn't work! Having a test that supports your diagnosis should be very helpful, especially for primary care docs. Also, you don't have to incur the costs of running a test to the lab, or worry about the lab rejecting it because of the patient's insurance, which has been a problem for us in the past."
"Maybe the most important thing," Dr. Tauber adds, "is that I can tell a patient in 10 minutes, 'You have adenovirus and you're contagious. Don't go back to work, because you're going to spread this.' Preventing an outbreak is critical, and this test should help do that."
Dr. Tauber only sees one potential downside to this technology. "Practices may get fewer patients as a result of primary-care docs having this test. This could have some effect on a practice's bottom line." Nevertheless, Dr. Tauber thinks it's a worthwhile trade-off. "If patients with conjunctivitis come in, they'll come in for more appropriate reasons, and we'll be better able to address their problem."
Coming Soon?
Dr. Sambursky says the clinical trials of the RPS adenovirus collector should be completed by May. Pending FDA approval, RPS hopes the adenovirus detector will be available by late summer.





