Tuberculosis is a global health challenge, affecting more than 2 billion people worldwide, with Asian countries like India and China being the hardest hit.1-4 Although primarily affecting the lungs, extrapulmonary involvement, including intraocular TB (IOTB), can occur in up to 20 percent of patients.5 The most common manifestation of IOTB is posterior uveitis, and the most common structure involved is the choroid.6,7,8 In this article, we’ll discuss ways to diagnose and manage this condition.
Etiology and Diagnosis
The disease has protean clinical manifestations, and its phenotypic expression can mimic several other inflammatory and non-inflammatory diseases that cause intraocular inflammation, making it difficult to diagnose and manage. The disease can present as:
• granulomatous anterior uveitis;
• intermediate uveitis;
• choroidal tuberculomas;
• serpiginous-like choroiditis;
• multifocal choroiditis;
• retinal vasculitis neuroretinitis;
• optic neuritis;
• panuveitis; and
• scleritis.
Rarely, IOTB may present as panophthalmitis, endophthalmitis, optic neuropathy or optic nerve tubercle. These uncommon phenotypes are mostly seen in endemic regions. The varied spectrum of presentation, coupled with the difficulty of making a definitive diagnosis, due to lack of a tissue sample and uncertain guidelines and protocols for anti-tuberculosis treatment, make both diagnosis and treatment a challenge.
A recently completed multicenter retrospective collaborative study (the Collaborative Ocular Tuberculosis Study [COTS-1]) collected data from 40 uveitis experts around the world in order to address the current controversies related to the diagnosis and management of intraocular tuberculosis.9-12 The study had 945 subjects, 74 percent of whom were Asian. The study showed a higher prevalence of intraocular tuberculosis in the Asian population, in both native residents and immigrants. Among the Asian patients, the most common manifestation was TB serpiginous-like choroiditis (SLC), which was far less common in the Western population.9 This study highlighted the variations in practice guidelines and lack of consensus among the experts about the diagnosis and management of this disease.
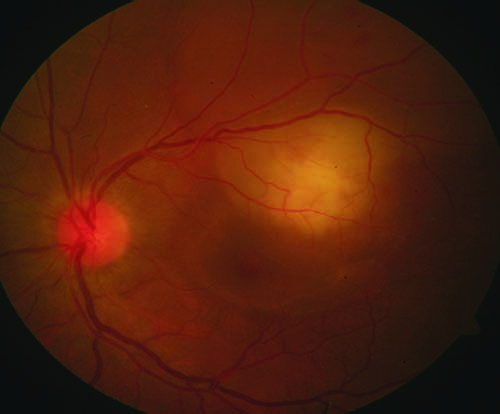 |
| Figure 1. Color fundus photograph of the left eye shows choroidal granulomas as subretinal, elevated yellowish lesions. |
Imaging Signs
Intraocular tuberculosis is usually suspected based on the clinical signs, and the diagnosis is further supported by ancillary and laboratory investigations. Ancillary investigations, including fundus autofluorescence, fluorescein angiography, indocyanine green angiography and optical coherence tomography, may detect characteristic features and are useful in detecting posterior segment disease.13
Choroidal tubercles are mostly located in the posterior pole or mid-periphery, and they show initial hypofluorescence in the early phases of FA, followed by hyperfluorescence in the later phases. These tubercles can also be seen as focal areas of choroidal elevation on OCT. Choroidal granulomas appear as subretinal yellowish lesions and may be associated with retinal detachment and subretinal fluid (Figure 1). The larger granulomas may form subretinal abscesses. Similar to choroidal tubercles, granulomas and abscesses are associated with early hypofluorescence and late hyperfluorescence. On ICGA, these appear hypofluorescent in both early and late stages. OCT in these lesions has become a helpful tool for detecting detachments as well as the location of granulomas. When compared with other pathologies, tubercular granulomas have a non-homogenous internal pattern with a lobulated shape.
Serpiginous-like choroiditis has three distinct patterns: multifocal; placoid; and mixed.14,15 The multifocal pattern is characterized by multiple discrete, yellowish lesions with well-defined margins. The placoid pattern appears as a large plaque with yellowish edges and pigmentary changes in the center. When both features are present, the disease is characterized as mixed.
When observed on FA, multifocal SLC lesions are hypofluorescent in early phases of the angiogram, with hyperfluorescence seen in late phases. Placoid lesions show mixed fluorescence in the center, with early hypofluorescence followed by late fluorescence on the edges. When SLC patterns are seen on fundus autofluorescence, early disease usually appears as hyperautofluorescent. As the lesion begins to heal, FAF shows a mixture of both hypo- and hyperautofluorescence, followed by an increase in hypoautofluorescence with a stippled intermixed pattern and finally a uniform hypoautofluorescence (Figures 2 to 4).16
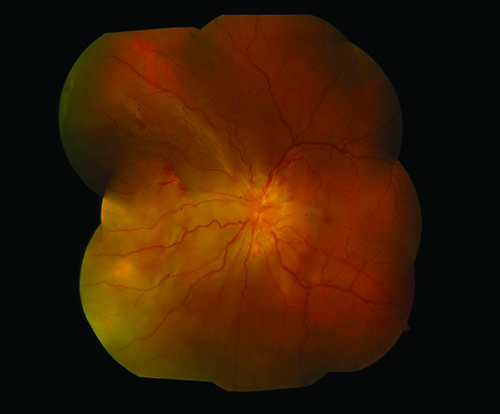 |
| Figure 2. Color fundus photograph of the left eye shows large nasal choroidal granulomas with exudative retinal detachment. |
Several newer imaging techniques like ultra widefield imaging can play a significant role in identifying peripheral lesions in SLC that may be otherwise missed by conventional imaging.17 OCT also helps detect retinochoroidal changes in TB SLC. OCT B-scans passing the active edge of TB SLC during the active stage show an area of hyper-reflectivity in the outer retinal layers with no increased backscattering from the inner choroid. As the lesions heal, the hyper-reflective fuzzy areas disappear and are replaced by irregular, hyper-reflective knobby elevations of the outer retinal layers and other retinal layers, including RPE, ellipsoid and myoid zones; the ELM can’t be identified at this stage.13 Choroidal vascularity index measurement of the active SLC on OCT will show a relative decrease in choroidal vascularity, with choriocapillaris atrophy and the remodeling of the choroid during the healing stage.18 OCT angiography has demonstrated novel findings that show voids in areas of choriocapillaris flow during the active stage of SLC that also correlate well with indocyanine green angiography.19 ICGA is also very useful for detecting choroidal neovascular membranes that may otherwise be missed.20
Presumed TB retinal vasculitis is peripheral occlusive retinal periphlebitis, with or without active retinal periphlebitis and features of peripheral capillary nonperfusion. Tubercular retinal vasculitis is mainly occlusive, and may result in peripheral neovascularization with recurrent vitreous hemorrhages, presenting as Eales’ disease. The presence of coexistent perivascular choroiditis lesions is suggestive of tubercular etiology.12
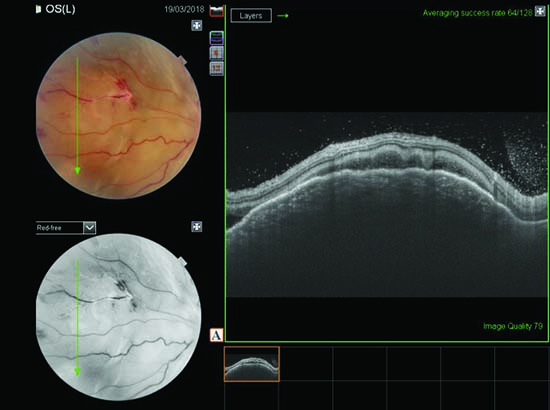 |
| Figure 3. OCT line scan passing through the granuloma shows elevation of the choroid. |
Testing and Treatment
There is no single, gold-standard test for diagnosing IOTB. The confirmatory tests to isolate Mycobacterium, such as a positive acid-fast bacillus test or a positive culture from the ocular fluid, are rarely possible. Thus, detection of the mycobacterial genome by positive polymerase chain reaction for IS 6110 is often tried as an alternative.21,22 However, the lack of standardization makes it difficult to recommend PCR as a diagnostic test.21 Tests like QuantiFERON TB gold, Purified Protein Derivative (PPD) and CT chest are corroborative at best.
So far, the stepladder approach to making the diagnosis of IOTB is widely followed. This approach involves identifying the clinical features and excluding other causes of uveitis, followed by looking at the results of a chest X-ray or computed tomography scan, PPD or QuantiFERON, and also considering intraocular sampling with molecular testing to confirm the diagnosis in certain situations.
Currently, there’s no consensus regarding treatment for IOTB due to a lack of guidelines regarding both initiation and duration of anti-tubercular drugs. The standard therapy for IOTB consists of isoniazid, rifampicin, ethambutol, pyrazinamide and pyridoxine. The therapy is initiated with 5 mg/kg/day of isoniazid, 10 mg/kg/day of rifampicin, 15 mg/kg/day of ethambutol, 20 to 25 mg/kg/day of pyrazinamide, and 10 mg/day of pyridoxine. The treatment should be started after carefully weighing the risks and benefits, along with monitoring liver function on a regular basis. There are no specific guidelines dictating the duration of treatment, and it’s usually given for six months, with discontinuation of ethambutol and pyrazinamide after two months. Therapy helps reduce disease recurrence, and the COTS study reported treatment success in nearly 87 percent of 801 patients who received anti-TB therapy for various indications.9
The paradoxical worsening of the disease after the initiation of treatment further contributes to the contentious role of anti-TB therapy (ATT) in IOTB.23 This phenomenon is most commonly observed in patients with TB SLC. Paradoxical worsening is observed due to a combination of multiple factors, including increased type IV hypersensitivity, increased exposure to Mycobacterium antigen such as tubercular proteins and the eye’s response to them, and decreased suppressor mechanisms. The rate of detection of this worsening has increased significantly since the introduction of ultra-wide-field retinal imaging. UWF imaging detects 36 percent cases of worsening after two to six weeks of initiation of ATT versus only 14 percent with conventional imaging.17 OCTA, too, has proven to be very useful in detecting paradoxical worsening by showing the alteration in choriocapillaris.24 When detected, these patients require a higher dose of steroids or additional methylprednisolone pulses intravenously. In cases of anterior uveitis, topical steroids can be administered. The addition of steroid-sparing immunosuppressants is based on the patient’s need for them, as well as the discretion of the uveitis specialist.25-30
Concomitant use of corticosteroids to manage inflammation of the eye is based on the hypothesis that the inflammation is secondary to a type IV hypersensitivity reaction to tubercular proteins. Steroids have also shown efficacy in reducing the macular edema secondary to the inflammation. Steroids are started with ATT with a dose of 1 mg/kg/day, which is then tapered over six to twelve months. Despite the supporting evidence, no one has defined clear guidelines specifying the use of corticosteroids, with certain authors supporting steroids’ role and a few favoring their use only in cases of macular involvement.
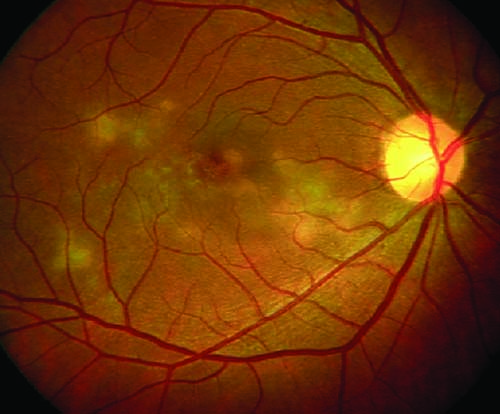 |
| Figure 4. Color fundus photograph of the right eye shows multifocal serpiginoid choroiditis. |
The use of novel agents to treat tuberculosis has been on the rise, mainly to address the adverse effects associated with conventional therapy, as well as the challenges associated with paradoxical worsening of the disease. Intravitreal injections of depot steroids have been shown to improve outcomes in patients worsening on steroid and ATT therapy.25-27 Other therapies proving to be effective as second-line treatments include intravitreal methotrexate and Interferon-alpha 2a.28-30
Treatment variability, including the use of concomitant steroids or immunosuppressants, is largely based on the standard clinical practice of a particular region. Treatment of IOTB is often managed with the help of a pulmonologist or an infectious disease specialist, following guidelines based on local practices. In COTS-1, researchers emphasized the difference in the outcome between patients from different ethnicities as well as geographic locations, with Asian patients having better overall survival outcomes after treatment.
Despite the advances in diagnostic tools for IOTB, clinical diagnosis remains a complex issue, and it influences the eventual treatment of the disease. To properly manage IOTB, the ophthalmologist and the internist have to work together, come to a consensus on the terminology they’ll use with regard to IOTB, establish guidelines for their clinical investigation and determine the best treatment course to pursue in endemic and non-endemic regions. REVIEW
Dr. Mahajan is a visiting instructor at Byers Eye Institute, Stanford Health Care in Palo Alto, where Dr. Nguyen is a professor of ophthalmology. Dr. Agrawal is an adjunct associate professor at the Tan Tock Seng Hospital in Singapore. Dr. Gupta is a professor in the department of ophthalmology at the Advanced Eye Centre, Post-graduate Institute of Medical Education and Research (PGIMER) in Chandigarh, India.
They have no financial interest in any products mentioned.
Direct correspondence to: Vishali Gupta, MS, Professor, Advanced Eye Center, Post Graduate Institute of Medical Education and Research (PGIMER), Sector 12, Chandigarh – 160012, India. Phone: +91 172 274 7837. Email: vishalisara@yahoo.co.in; vishalisara@gmail.com.
1. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;282:677–686.
2. Chakraborty AK. Epidemiology of tuberculosis: Current status in India. Indian J Med Res 2004;120:4:248–276.
3. Chadha VK. Tuberculosis epidemiology in India: A review. Int. J. Tuberc Lung Dis 2005;9:10:1072–1082.
4. GBD Tuberculosis Collaborators. The global burden of tuberculosis: Results from the Global Burden of Disease Study 2015. Lancet Infect Dis 2018;18:3:261–284.
5. Shakarchi FI. Ocular tuberculosis: Current perspectives. Clin Ophthalmol 2015;9:2223–7. doi: 10.2147/OPTH.S65254. (Epub ahead of print)
6. Gupta V, Shoughy SS, Mahajan S, Khairallah M, Rosenbaum JT, Curi A, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm 2015;23:1:14–24.
7. Gupta V, Gupta A, Rao NA. Intraocular tuberculosis—an update. Surv Ophthalmol 2007;52:6:561–587.
8. Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol 2010;149:4:562–570.
9. Agrawal R, Gunasekeran DV, Grant R, Agarwal A, Kon OM, Nguyen QD, et al. Clinical features and outcomes of patients with tubercular uveitis treated with antitubercular therapy in the Collaborative Ocular Tuberculosis Study (COTS)-1. JAMA Ophthalmol 2017;135:12:1318–1327.
10. Agrawal R, Gunasekeran DV, Agarwal A, et al. The Collaborative Ocular Tuberculosis Study (COTS)-1: A Multinational Description of the Spectrum of Choroidal Involvement in 245 Patients with Tubercular Uveitis. Ocul Immunol Inflamm 2018 Aug 29:1-11. doi: 10.1080/09273948.2018.1489061 (Epub ahead of print)
11. Agrawal R, Gunasekeran DV, Raje D, Agarwal A, Nguyen QD, Kon OM, Pavesio C, Gupta V; Collaborative Ocular Tuberculosis Study Group. Global variations and challenges with tubercular uveitis in the collaborative ocular tuberculosis study. Invest Ophthalmol Vis Sci 2018;59:10:4162-4171.
12. Gunasekeran DV, Agrawal R, Agarwal A, et al; COTS-1 Study Group. The Collaborative Ocular Tuberculosis Study (COTS)-1: A multinational review of 251 patients with tubercular retinal vasculitis. Retina 2018 Apr 24. [Epub ahead of print]
13. Agarwal A, Mahajan S, Khairallah M, et al. Multimodal imaging in ocular tuberculosis. Ocul Immunol Inflamm 2017;25:1:134-145.
14. Gupta V, Gupta A, Arora S, et al. Presumed tubercular serpiginous-like choroiditis: Clinical presentations and management. Ophthalmology 2003;110:9:1744-9.
15. Bansal R, Gupta A, Gupta V, et al. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology 2012;119:11:2334-42.
16. Gupta A, Bansal R, Gupta V, Sharma A. Fundus autofluorescence in serpiginous-like choroiditis. Retina 2012;32:4:814-25.
17. Aggarwal K, Agarwal A, Deokar A, et al. Ultra-wide field imaging in paradoxical worsening of tubercular multifocal serpiginoid choroiditis after the initiation of anti-tubercular therapy. Ocul Immunol Inflamm. 2017;11:1-6.
18. Agarwal A, Agrawal R, Khandelwal N, et al. Choroidal structural changes in tubercular multifocal serpiginoid choroiditis. Ocul Immunol Inflamm 2018;26:6:838-844.
19. Mandadi SKR, Agarwal A, Aggarwal K, et al; the OCTA Study Group. Novel findings on optical coherence tomography angiography in patients with tubercular serpiginous-like choroiditis. Retina 2017;37:9:1647-1659.
20. Aggarwal K, Agarwal A, Sharma A, Sharma K, Gupta V; OCTA Study Group. Detection of Type 1 choroidal neovascular membranes using OCTA in tubercular posterior uveitis. Retina 2018 Apr 23. [Epub ahead of print]
21. Agarwal A, Agrawal R, Gunasekaran DV, et al. The Collaborative Ocular Tuberculosis Study (COTS)-1 report 3: Polymerase chain reaction in the diagnosis and management of tubercular uveitis: Global trends. Ocul Immunol Inflamm 2017 Dec 20:1-9. Doi 10.1080/09273948.2017.1406529. [Epub ahead of print]
22. Sharma K, Gupta V, Sharma A, et al. Gene Xpert MTB/RIF assay for the diagnosis of intra-ocular tuberculosis from vitreous fluid samples. Tuberculosis (Edinb) 2017;102:1-2.
23. Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating anti-tuberculosis treatment. Am J Ophthalmol 2011;152:5:857-863.
24. Agarwal A, Aggarwal K, Deokar A, et al. Optical coherence tomography angiography features of paradoxical worsening in tubercular multifocal serpiginoid choroiditis. Ocul Immunol Inflamm 2016;24:6:621–630.
25. Fonollosa A, Valsero S, Artaraz J, Ruiz-Arruza I. Dexamethasone intravitreal implants in the management of tubercular multifocal serpiginoid choroiditis. J Ophthalmic Inflamm Infect 2016;6:1:31.
26. Jain L, Panda KG, Basu S. Clinical outcomes of adjunctive sustained-release intravitreal dexamethasone implants in tuberculosis-associated multifocal serpigenoid choroiditis. Ocul Immunol Inflamm 2018;26:6:877-883.
27. Agarwal A, Handa S, Aggarwal K, et al. The role of dexamethasone implant in the management of tubercular uveitis. Ocul Immunol Inflamm 2018;26:6:884-892.
28. Julian K, Langner-Wegscheider B-J, Haas A, De Smet MD. Intravitreal methotrexate in the management of presumed tuberculous serpiginous-like choroiditis. Retina 2013;33:9:1943.
29. Sahin O, Ziaei A. The role of methotrexate in resolving ocular inflammation after specific therapy for presumed latent syphilitic uveitis and presumed tuberculosis-related uveitis. Retina 2014;34:7:1451–1459.
30. La Distia Nora R, Walburg KV, van Hagen PM, et al. Retinal pigment epithelial cells control early Mycobacterium tuberculosis infection via interferon signaling. Invest Ophthalmol Vis Sci 2018;59:3:1384–1395.
31. Invernizzi A, Iannaccone F, Marchi S, et al. Interferon alpha-2a for the treatment of post-infectious uveitis secondary to presumed intraocular tuberculosis. Ocul Immunol Inflamm 2018 Feb 22:1-8. doi: 10.1080/09273948.2018.1431292. (Epub ahead of print)



