These new systems all feature 24-inch, high-resolution, lightweight LED displays. Reichert says they come equipped with an easy-to-navigate interface controlled from the Phoroptor VRx Digital Refraction System, SightChek Digital Phoroptor or a simple infrared remote. The systems also require no separate components or software.
The ClearChart 4X and ClearChart 4P allow users to import images and videos from a USB drive. They also include ETDRS, contrast sensitivity and patient education slides. All models can be configured for standard or mirrored viewing for refracting distances from 6 to 24.6 feet.
For more information on Reichert’s ClearChart systems, visit reichert.com/clearchart.
Bausch + Lomb Introduces Vitesse
In late April, Bausch + Lomb announced that Vitesse, a hypersonic, completely open-port vitrectomy system, received FDA clearance. This news follows close behind the company’s FDA approval for its Stellaris Elite Vision Enhancement System. Vitesse will be available exclusively on the Stellaris Elite in the coming year.
Vitesse’s design consists of a single lumen with a fixed, open port for consistent flow. Bausch + Lomb says that this design creates a highly localized tissue liquefication zone, which liquefies the vitreous at the edge of the port before aspiration. The company hopes that this new technology will eliminate the traditional “guillotine-style” vitrectomy cutter, which was introduced 40 years ago.
For more information on Bausch + Lomb’s Vitesse and Stellaris Elite, visit bausch.com/our-products.
D-EYE Upgrades System
In late April, D-EYE released an upgrade to its
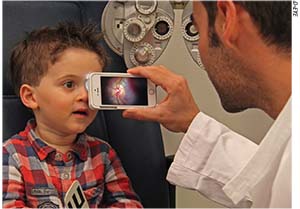 |
D-EYE also recently introduced a LAN share feature that allows images and videos to be moved across a local area network to Window’s PCs and Macs.
For more information on Image-Select, visit d-eyecare.com.
Haag-Streit Fundus Module 300
Haag-Streit recently announced it received FDA approval for its Fundus Module 300 slit lamp attachment. It attaches directly to the slit lamp for integration into the examination process, unlike handheld fundus camera devices, the company says.
The camera is controlled with Haag-Streit’s control panel (RM02), and images captured by the device are transferred to Haag-Streit’s EyeSuite software, allowing for more convenient and efficient examinations for both physicians and their patients, Haag-Streit says. It is compatible with the BQ 900, BP 900, BI 900 and BM 900 slit lamps. It can also be used in combination with the IM 900 or IM 600.
For more information on Haag-Streit’s Fundus Module 300, visit hsdriven.com/fundus.
Oasis Debuts Punctal Plug
Oasis recently added a new punctal plug to its Soft Plug punctal occlusion line: the Soft Plug Extended Duration 180. It’s made of an absorbable polydiozanone material and can last up to 180 days. The new plugs come in three sizes: 0.3 x 2.0 mm; 0.4 x 2.0 mm; and 0.5 x 2.0 mm.
In addition to the plugs, Oasis recently added its Lid & Lash pre-soaked pads, available with or without Tea Tree Oil, to its line
 |
For more information on OASIS Medical’s Punctal Plug and Eyelid pads, visit oasismedical.com.
Allergan TrueTear
Allergan announced that the FDA recently approved its TrueTear Intranasal Tear Neurostimulator, the first and only FDA-cleared device that temporarily increases tear production in a neurostimulation.
The handheld stimulator uses daily disposable tips that are inserted into the nasal cavity to induce tear production through neurostimulation. Allergan says two clinical studies showed positive safety and efficacy of the device in 145 aqueous-deficient dry-eye patients.
For more information on the True-Tear or the two clinical studies, visit allergan.com.
Optovue’s High-Density OCT Angiography
By releasing AngioVueHD Imaging, Optovue says it has become the first company to offer higher density OCTA imaging that improves resolution and peripheral visualization of vasculature in the eye. Optovue claims that higher resolution and increase in sampling points will enable physicians to more closely study the fine vasculature in the eye for changes that could indicate ocular disease.
According to the company, the standard field-of-view for the best image quality measures 3 x 3 mm, while the AngioVueHD update allows equivalent image quality in the 6 x 6 mm field-of-view.
Optovue has also recently released the AngioVueHD Montage, which combines two high-density images (one at the central macular region and the other at the optic disc), resulting in a 10 x 6 mm field of view. The company says this is useful for imaging vasculature in potential pathologies that may extend into the periphery of the imaging plane.
For more information on the AngioVueHD and AngioVueHD Montage, visit optovue.com.
Lucentis Approved for DR
Genentech’s Lucentis ranibizumab injection 0.3 mg is now approved for the monthly treatment of all forms of diabetic retinopathy, making it the only drug with this distinction.
The injection received FDA approval based on an analysis of the NIH-funded Diabetic Retinopathy Clinical Research Network’s Protocol S study, in which Lucentis was compared to panretinal laser treatment in diabetic retinopathy patients with and without diabetic macular edema. Patients both with and without DME in the Lucentis group experienced improvements in the severity of their retinopathy.
For more information on Lucentis, visit gene.com/patients/medicines/lucentis.



