Boston researchers have identified a way to enhance regrowth of human corneal tissue to restore vision, using a molecule known as ABCB5 that acts as a marker for hard-to-find limbal stem cells. This work, a collaboration between the Massachusetts Eye and Ear/Schepens Eye Research Institute, Boston Children’s Hospital, Brigham and Women’s Hospital and the VA Boston Healthcare System, provides promise to burn victims, victims of chemical injury and others with damaging eye diseases. The research, published this week in
Nature, is also one of the first known examples of constructing a tissue from an adult-derived human stem cell.
Limbal stem cells help maintain and regenerate corneal tissue. Their loss due to injury or disease is one of the leading causes of blindness. In the past, tissue or cell transplants have been used to help the cornea regenerate, but it was unknown whether there were actual limbal stem cells in the grafts, or how many, and the outcomes were not consistent.
In this study, researchers were able to use antibodies detecting ABCB5 to zero in on the stem cells in tissue from deceased human donors and use them to regrow anatomically correct, fully functional human corneas in mice.
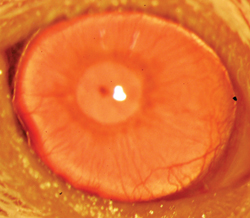 |
|
A restored functional cornea following transplantation of human ABCB5-positive limbal stem cells to limbal stem cell-deficient mice. Transplants consisting of human ABCB5-positive limbal stem cells resulted in restoration and long-term maintenance of a normal clear cornea, whereas control mice that received either no cells or ABCB5-negative cells failed to restore the cornea. (
Image courtesy K Lathrop, B Ksander, M Frank and N Frank.) |
|
“Limbal stem cells are very rare, and successful transplants are dependent on these rare cells,” says Bruce Ksander, PhD, of Mass Eye and Ear, co-lead author on the study with post-doctoral fellow Paraskevi Kolovou, MD. “This finding will now make it much easier to restore the corneal surface. It’s a very good example of basic research moving quickly to a translational application.”
ABCB5 was originally discovered in the lab of Markus Frank, MD, of Boston Children’s Hospital, and Natasha Frank, MD, of the VA Boston Healthcare System and Brigham and Women’s Hospital, co-senior investigators on the study, as being produced in tissue precursor cells in human skin and intestine. In the new work, using a mouse model developed by the Frank lab, they found that ABCB5 also occurs in limbal stem cells and is required for their maintenance and survival, and for corneal development and repair. Mice lacking a functional ABCB5 gene lost their populations of limbal stem cells, and their corneas healed poorly after injury.
“ABCB5 allows limbal stem cells to survive, protecting them from apoptosis [programmed cell death],” said Markus Frank. “The mouse model allowed us for the first time to understand the role of ABCB5 in normal development, and should be very important to the stem cell field in general,” said Natasha Frank.
Markus Frank is working with the biopharmaceutical industry to develop a clinical-grade ABCB5 antibody that would meet U.S. regulatory approvals. “A single lab cannot do a study like this,” said Natasha Frank, also affiliated with the Harvard Stem Cell Institute. “It integrates genetics, knockout mice, antibodies, transplantation—a lot of technical expertise that we were lucky came together in a very nice way.”
|
FDA Approval for Ozurdex 0.7 mg for Select DME Cases |
|
The Food and Drug Administration approved Allergan’s Ozurdex (dexamethasone intravitreal implant) as a new treatment option for diabetic macular edema in adult patients who have an artificial lens implant or who are scheduled for cataract surgery. Ozurdex is a sustained-release biodegradable steroid implant that demonstrated long-term efficacy without the need for monthly injections.
DME currently impacts more than 560,000 Americans. The Ozurdex implant uses the proprietary and innovative Novadur solid polymer delivery system—a biodegradable implant that releases medicine over an extended period of time—to suppress inflammation, which plays a key role in the development of DME.
The FDA approval of Ozurdex for this indication is based on the MEAD (Macular Edema: Assessment of Implantable Dexamethasone in Diabetes) study. MEAD includes two multicenter, three-year, sham-controlled, masked, randomized clinical studies assessing the proportion of patients with 15 or more letters improvement in best-corrected visual acuity from baseline. The most common adverse events in the studies included cataracts and elevated intraocular pressure. An increase in mean IOP was seen with each treatment cycle, and the mean IOP generally returned to baseline between treatment cycles.
“DME is a complicated disease to treat,” said Pravin Dugel, MD, clinical associate professor of ophthalmology at the Keck School of Medicine at the University of Southern California, managing partner of Retinal Consultants of Arizona, and clinical investigator in the MEAD clinical trial. “Ozurdex provides long-term improvement of DME without the need for monthly injections, which helps these patients who are also managing the other conditions common with diabetes.”
The Ozurdex implant is already indicated for the treatment of macular edema following branch retinal vein occlusion or central retinal vein occlusion and for the treatment of non-infectious uveitis affecting the posterior segment of the eye. |
|
|
Cataract Surgery Pays Dividends in Alzheimer’s Patients
Cataract surgery for people with Alzheimer’s disease and other dementias not only improves vision but can slow decline in cognition and improve quality of life for both people with the disease and their caregivers, according to clinical trial results reported in July at the Alzheimer’s Association International Conference 2014 in Copenhagen, Denmark.
“This study supports the Alzheimer’s Association view that people with dementia retain, and benefit from, full health-care treatment,” said Maria Carrillo, PhD, Alzheimer’s Association vice president of medical and scientific relations. “Too common attitudes such as, ‘There’s no need for extra care’ or ‘Why put them through all of that’ are not justified and are bad medical practice.”
“Appropriate thoughtfulness and restraint are necessary when considering surgery or other procedures for people with Alzheimer’s or another dementia. However, we should not assume that medical procedures cannot be pursued or are too risky. As these new results show, improving sensory abilities, for example, can provide benefits in a variety of ways—for people with Alzheimer’s and also for their caregivers from whom unnecessary burden can be lifted,” Dr. Carrillo said.
|
Expanded Approval for B + L Victus |
|
Bausch + Lomb has received 510(k) clearance from the FDA for the Victus Femtosecond Laser Platform for laser-assisted lens fragmentation during cataract surgery.
The fragmentation procedure, which follows a capsulotomy, uses the femtosecond laser to split the cataractous lens into sections. This is followed by phacoemulsification for cataract removal. he Victus platform offers a number of different lens fragmentation patterns depending on the cataract grade and user preference.
“Academic research has shown that cataracts pre-treated with lens fragmentation can require less phacoemulsification energy for removal,” said Y. Ralph Chu, MD, founder and director of the Chu Vision Institute, Bloomington, Minn. “In lower grade cataracts, we have seen up to a 50-percent reduction in the phaco energy required to remove the lens following lens fragmentation with the laser, compared with standard phaco.”
B + L has been installing Victus platforms in leading surgery centers globally since it received CE mark in November 2011 and the FDA clearances in July 2012. It is now one of the only femtosecond lasers in the U.S. with clearance for the creation of a corneal flap in patients undergoing LASIK surgery, anterior capsulotomy during cataract surgery, penetrating arcuate cuts/incisions in the cornea and laser-assisted lens fragmentation during cataract surgery. |
|
|
At AAIC 2014, Alan J. Lerner, MD, of Case Western Reserve University and University Hospitals Case Medical Center and colleagues reported interim results from an ongoing clinical trial to determine the effects of cataract removal on several measures of visual ability, cognitive measures, and quality of life in people with dementia. Study participants are recruited from dementia and ophthalmology clinics at University Hospitals Case Medical Center and MetroHealth Medical Center in Cleveland, and are divided into two groups: 1) immediate surgery following recruitment and; 2) delayed or refused surgery. Vision and cognitive status, mood and capability to complete daily activities are evaluated at baseline and six months after recruitment, or six months after surgery.
Preliminary analysis of results from 20 surgical and eight non-surgical participants showed that the surgical group had significantly improved visual acuity and quality of life, reduced decline in memory and executive functioning, and improvements in behavioral measures compared with the non-surgical group. Levels of perceived burden for caregivers of people in the surgical group also showed improvement.
“These preliminary results indicate that improved vision can have a variety of benefits for people with dementia and their loved ones, both visual and non-visual,” said Dr. Lerner. “Our findings need to be verified in a larger study, but they suggest the need to aggressively address dementia co-morbidities such as vision-impairing cataracts, while balancing safety and medical risks.”
“If the results hold up, it will significantly affect how we treat cataracts in individuals with dementia. Other interventions to offset sensory loss—including vision and hearing—may help improve quality of life for people with dementia and their caregivers,” Dr. Lerner added.
According to the Alzheimer’s Association, a person with dementia has the right to any medical treatment available. People with the disease may require longer courses of some treatments such as rehabilitative therapies compared to people with intact cognition. Therapies that may be of benefit should not be discontinued because a person with Alzheimer’s has failed to make progress as the same rate as someone without the disease.
Making medical decisions about treatment remains the right of the person with Alzheimer’s until he or she no longer has the cognitive capacity to understand the decision. At that time, medical decisions are made by the person’s surrogate. The Alzheimer’s Association recommends that preferences about medical treatment and decisions should be addressed early in the disease process through the execution of advance directives. Absent an advance directive, the surrogate decision maker should be guided by the values and any expressed wishes of the person with Alzheimer’s disease.
REVIEW




