Today’s patient population of irregular astigmats differs from 10 or 15 years ago, when the bulk of cases were caused by previous refractive surgery. “In the early days, the laser technology wasn’t as good and there were misconceptions about eye registration,” says Aleksandar Stojanovic, MD, PhD, medical director of SynsLaser Clinic in Tromsø and Oslo, Norway. “For example, hyperopes usually have a big discrepancy between the center of the pupil and optical center of the cornea. As a rule, patients use their optical center for vision, so centering the laser on the center of the pupil caused irregular optics for those eyes. This generated a large number of irregular astigmatism cases. Now, we tend to see more ectatic causes.”
Here, experts break down irregular astigmatism management, from making the diagnosis to performing off-label topography-guided PRK.
Preference for Topo-guided
 |
|
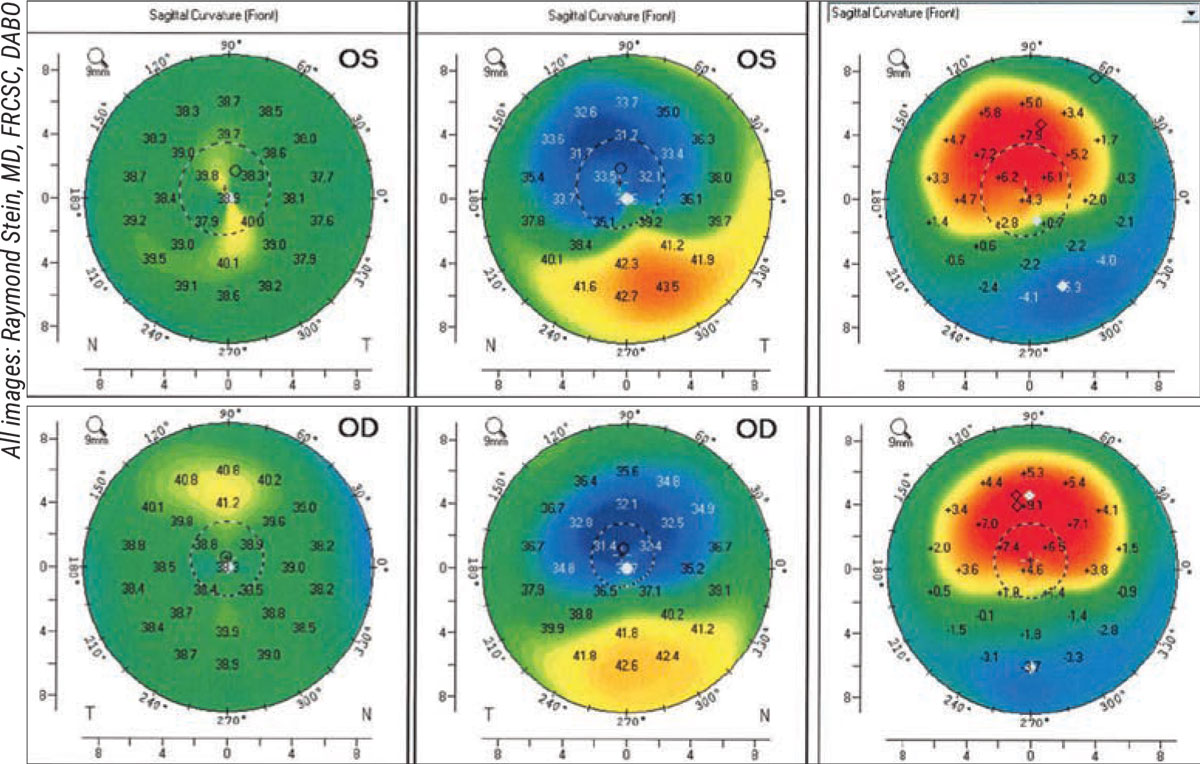
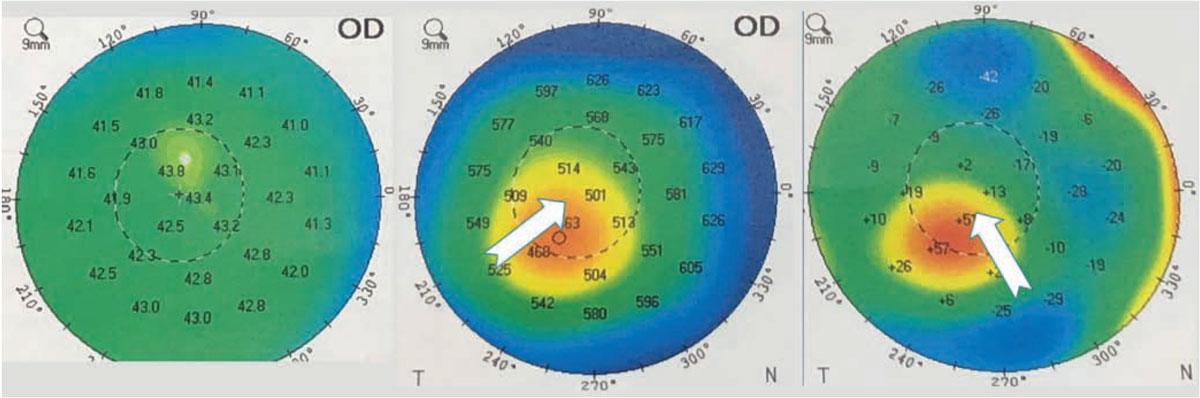
Figure 1. Postop (left), preop (center) and difference maps (right) of an atypical case involving a 32-year-old male who underwent TG-PRK for significant irregular astigmatism prior to left-eye cataract surgery. The patient had previously undergone LASIK OU at age 20 and wore scleral lenses for irregular astigmatism. His BCVA was 20/40 OD and 20/80 OS. Corneal thickness was 443 µm OD and 428 µm OS. The TG-PRK induced significant myopia by steepening the central cornea, as seen in the difference maps. Six months after the ablation when the topography was stable, the patient underwent cataract surgery and received an aspheric toric implant aimed at -2.5 D. At one week postop, the patient’s visual acuity was J2 (-2.25 -0.25x90 20/20). |
Dr. Stojanovic, the ophthalmologist responsible for introducing LASIK and cTEN to Norway, says he uses both placido-disc-guided and Scheimpflug-guided topography for treating irregular astigmatism. “My American colleagues use TG-PRK as well, always off-label, because there’s no other way to correct the corneal optics,” he says. “The aim of TG-PRK is primarily to correct the astigmatism to the degree that the patient isn’t bothered by visual disturbances, and to increase as much as possible their corrected visual acuity. Wavefront-guided isn’t good enough because we don’t get enough data to base our treatment on.”
Most surgeons prefer topography-guided to wavefront-guided treatments in irregular corneas for this very reason. “Most wavefront-guided machines measure about 1,200 to 1,300 data points, whereas topography-guided machines can measure more than 20,000 data points,” explains Raymond Stein, MD, FRCSC, medical director of the Bochner Eye Institute in Toronto, Ontario, and professor of ophthalmology and vision sciences at the University of Toronto. “This means less interpolation between the collected data points, giving us greater treatment potential. We can address variations in corneal elevation more precisely by flattening steep areas and steepening flat areas by certain amounts. This spares tissue, which is important since we’re often dealing with corneas of abnormal thickness.”
He adds that unlike in standard PRK, iris or limbal registration is used in topography-guided treatments. “This can be very helpful in terms of compensating for cyclotorsion, ensuring the treatment is exactly as the surgeon intends,” he says.
Is It Ectasia?
Distinguishing between ectatic conditions (e.g., keratoconus, pellucid marginal degeneration or ectasia after LVC) and non-ectatic conditions is key, says Dr. Stein. He explains that non-ectatic conditions such as EBMD and Salzmann’s nodular degeneration are contraindications for TG-PRK, because performing TG-PRK on a non-ectatic eye can induce more corneal irregularity. “Patients with pseudokeratoconic presentations secondary to a non-ectatic condition typically have a normal stroma, with only an irregular epithelium,” he says. “If you go in with TG-PRK, once you remove the epithelium, you’ll induce irregularity into the cornea.
“Instead, we treat EMBD and Salzman’s with a superficial keratectomy, which is essentially epithelial debridement,” he says. “We remove the irregular epithelium; this can regularize the cornea and improve vision. Other secondary causes such as a superficial punctate keratopathy secondary to dry eye or a blepharokeratitis are treated aggressively, usually with lid management and preservative-free artificial tears.”
He stresses careful interpretation of topographic maps to make the correct diagnosis. “Identifying the etiology requires corneal topography or tomography and a complete ophthalmic examination,” he says. “Anterior curvature changes without significant posterior elevation on tomography are suggestive of non-ectatic corneas. Slit lamp exams may also reveal the underlying cause of corneal steepening.”
Astigmatic Origins
Dr. Stojanovic says a high-quality preoperative exam is the most important part of the procedure, followed by evaluating the results and knowing how to use them. “The actual ablation is very straightforward,” he says.
Astigmatism is complex. “You need to know where the astigmatism comes from,” he explains. “Your approach will depend on the origin. Additionally, we need to know the higher order aberrations influencing the patient’s manifest refraction. We can’t treat only the astigmatism measured on the exam and then treat the HOAs. We must use the astigmatism measured amidst the HOAs in a mixture of totals, so we can isolate the astigmatism component from the HOAs. Using wavefront aberrometry to get the optics for the whole eye, we can then subtract lenticular optics and look only at corneal optics.”
Robert M. Kershner, MD, MS, FACS, a professor and chairman of the Department of Ophthalmic Medical Technology at Palm Beach State College and president and CEO of Eye Laser Consulting Global in Palm Beach Gardens, Florida, points out that just because aberrations are present doesn’t necessarily mean they need correcting, if the patient’s functional vision is good. “It’s never as simple as creating a perfect optical system so the patient sees clearly,” says Dr. Kershner. “I’ve examined Air Force pilots with perfect vision and found their optical systems chock full of aberrations. Sometimes correcting the irregularities we see isn’t beneficial to the patient’s optical outcome. We also need to be careful about where those aberrations are.
“When you do a Shack-Hartmann wavefront analysis and look at the various levels of HOAs, you can prove that these optical aberrations are present. What you don’t know is where in the optical system they reside,” he explains. “The shape of the fundus, the anterior and posterior lens, the inner and outer cornea and the scleral shell may all be contributing to the aberrations. In phakic patients, it’s very difficult to parse out what’s lenticular and what’s fundus.
“Typically when we measure astigmatism on the cornea and compare it to refractive astigmatism, we find that the cornea can reflect a higher degree of astigmatism in the central 3-mm than the patient actually has optically,” he adds. “However, there’s no absolute correlation between the two.”
Planning Ablations
|
|
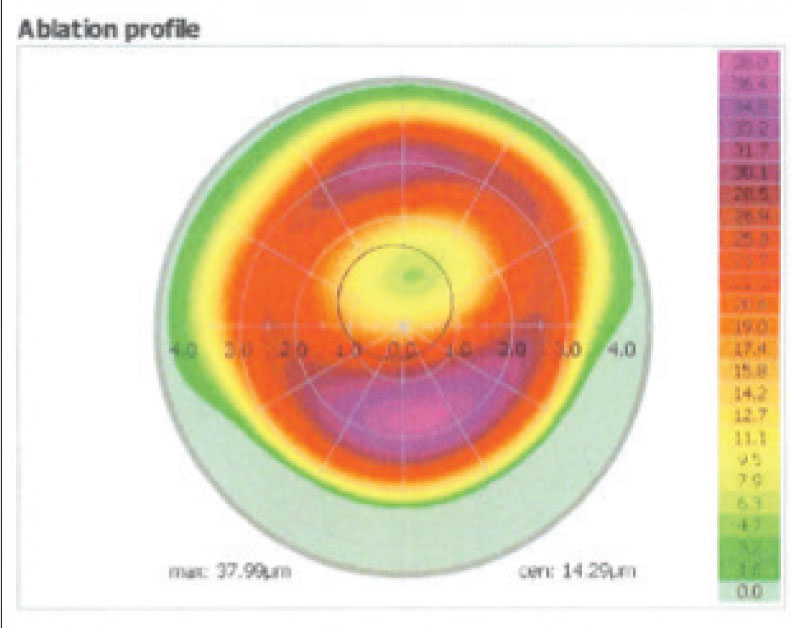
The ablation profile OD of the patient in Figure 1. |
Topography-guided technology has given surgeons access to many more corneal details than before. Using Alcon’s Allegretto excimer laser system, Dr. Stein says he has two options for ablation planning. “One is with a standard software with the Oculyzer II (WaveLight GmbH, Germany). With this approach, we take eight color images of the cornea and review them. If they’re all good-quality, we take the average and input this data into the laser. The difficulty with this standard treatment is that we never know how much myopia, hyperopia or astigmatism we may induce when we do TG-PRK.
“The other way we plan ablations is with a newer software developed for Contoura (Alcon) by ophthalmologist Mark Lobanoff, MD, in Minneapolis,” he continues. “The Phorcides Analytic Engine accounts for the change in refractive error just by doing the TG-PRK. We can use this in relatively normal corneas as well, for standard and topography-guided LASIK and PRK. The data for this software has shown outstanding outcomes in reducing mild degrees of irregular astigmatism.”
After imaging the cornea, Dr. Stein uses either software to develop a treatment protocol with a certain optical zone. “Generally we like to use a 6- or 6.5-mm optical zone,” he says. “Occasionally, we’ll have to shrink the optical zone to 5.5 or 5 mm, but if we do that, there’s a greater chance of regression. In general, we can use a large optical zone in patients with a dioptric difference of 10 D or less who also have corneas thicker than 460 µm at the thinnest point. We’ve found larger optical zones to be more effective at reducing irregular astigmatism.”
For modern LVC, the optical zone diameter typically falls in the 6- to 6.5-mm range. “The optical zone should be large enough to go beyond the borders of the pupil in low-light situations,” Dr. Kershner says. “When we first began doing refractive surgery, before LASIK, most optical corrections fell into the 5- to 5.5-mm zone, because we assumed the pupil wasn’t going to get much larger than that, except pharmacologically. For most cases that worked.
“Newer lasers now have computer algorithms that can do a blend, which tends to ablate a little more in the center and less in the periphery to maintain the asphericity that’s typical of a human cornea,” he explains. “If you do that, it lessens but doesn’t entirely eliminate the optical aberrations. You’re still doing a refractive correction, i.e., removing tissue within the visual axis, and the element of concern here is the pupil size.”
Low-light situations can dilate a blue-eyed person’s pupil up to 6 or 7 mm—which exceeds the typical optical correction zone—leaving them with unsatisfactory night vision and symptoms such as fuzziness, halo or double images. “A darker pigmented individual might not be as conscious of these problems at night because their pupils don’t dilate as much,” Dr. Kershner says. “That’s one of the drawbacks of surface ablation or excimer laser ablation with LASIK: It’s not a perfect process when you have to remove tissue from the center of the cornea and flatten it. It’s important to inquire about the importance of nighttime vision. Many patients won’t think to share this information unless you ask them directly.”
Surgery Day
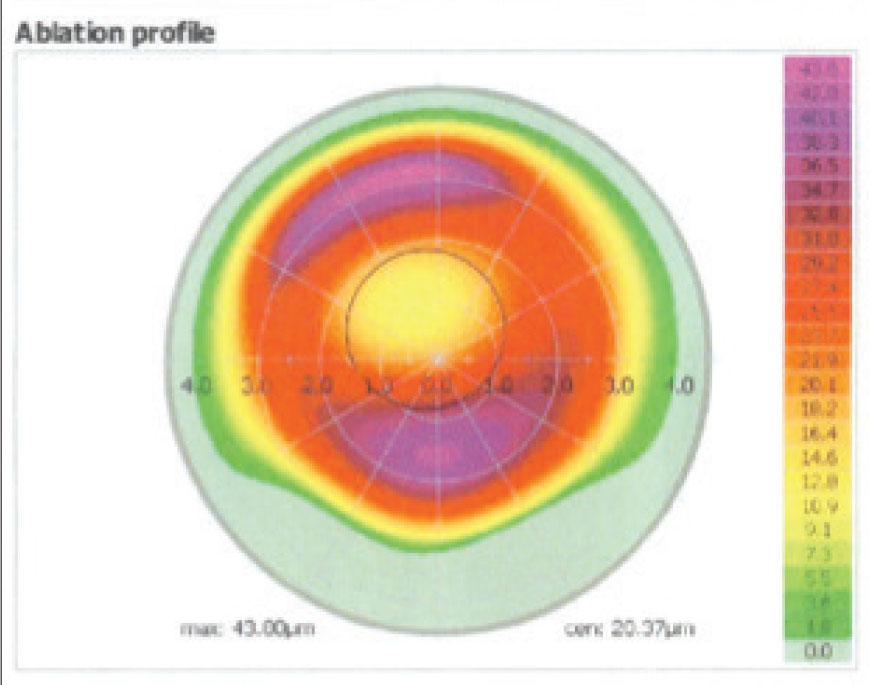
|
|
The ablation profile OS of the patient in Figure 1. |
Dr. Stein’s patients undergo imaging and refraction on the day of surgery, but he remarks that it’s important for contact lens wearers to be out of their contacts for an extended period prior to imaging, so the cornea can revert to its natural shape. He advises a washout period of at least a month for hard lenses, but sometimes longer depending on the number of years of wear. For soft contact lenses, he says one week is typical.
After identifying the optical zone he uses for the ablation, often a 6.5-mm zone with a transition zone to 8.3 mm, he removes the corneal epithelium with a 50-µm phototherapeutic keratectomy. “We typically limit the stromal ablation to 50 µm to allow enough residual thickness for a cross-linking procedure,” he says. “The combination of TG-PRK and CXL has a good chance at reducing irregular astigmatism. However, for patients with irregular astigmatism secondary to radial keratotomy, we tend not to perform CXL with TG-PRK. The success of CXL at decreasing diurnal vision fluctuations has been highly variable in these eyes.”
After the laser ablation, Dr. Stein applies ice to the cornea. “We’ve found this significantly reduces patients’ postoperative pain,” he says. He uses a frozen sponge soaked in BSS as a “popsicle” and applies this to the cornea for 10 seconds.
Dr. Stein then dries the cornea and applies a disc containing mitomycin-C for all of his PRK patients, whether they received standard PRK or TG-PRK. “We apply this for about one minute and then remove the disc,” he says. “We’ve found the MMC decreases the incidence of postoperative corneal haze. Then we irrigate the cornea with balanced saline to remove the MMC. If CXL is indicated, we perform it. Then we instill prednisolone acetate 1% and an antibiotic drop such as moxifloxacin 0.5%. We follow this with a bandage soft contact lens.”
After treatment, he has the patient wait in a recovery room for 10 to 15 minutes before undergoing a slit lamp examination to ensure the contact lens fits properly. “We like to see very little movement of the contact lens,” Dr. Stein says. “If it’s too loose, the patient will be uncomfortable.”
He sends the patient home with a nonsteroidal drop for the first 48 hours, an antibiotic drop to be administered four times daily until the contact lens comes out and a four-times-daily steroid drop, with the dose tapered over one month. He sees the patient back the following day, five days later to remove the bandage contact lens, and then typically at one, three and six months. “At six months we’ll often repeat the tomography, although the epithelium may not be totally mature and changes can occur even at one year,” he notes.
Follow-up
How you define a successful procedure sometimes depends in large part on the patient’s happiness. “The vital sign of the eye is: Can you see or can you not?” Dr. Kershner says. “Simply doing a Snellen acuity test doesn’t tell you much. It’s not a real-life situation, such as sitting in a dark room looking at a jet-black letter on a white surface. Real life is potential glare from car headlights or reading small text on a computer screen.”
He advises using all modalities of functional vision testing—in particular, contrast sensitivity and visual acuity under varying light conditions—to ensure that the patient sees well in a variety of real-life situations.
“There’s no fooling the patient,” he says. “You could achieve a perfect result and correct all of their refractive error and completely neutralize their topographic and wavefront maps, but if the patient can’t read the chart or can’t read a menu under dim lighting, it’s not a win. If they can read the chart and see clearly and function normally, then it was a success, even if you left them with some uncorrected astigmatism. The goal isn’t 20/20, it’s 20/happy.”
Annual follow-up exams that include tomography are key for ensuring the cornea has been regularized and stays that way after TG-PRK. Dr. Stein says that patients should undergo imaging, no matter what their level of UCVA. “You can’t see early ectasia at the slit lamp,” he points out. “It’s rare that a patient will require a retreatment due to progressive ectasia, but it’s a real concern. If the clinician picks it up at an early stage, you can save the patient’s sight by doing corneal cross-linking.”
Tomographic imaging is especially important for cases of irregular astigmatism secondary to keratoconus, pellucid marginal degeneration or ectasia after LVC. “We make sure that the cornea isn’t steepening in any area, especially in the area that was steep before,” says Dr. Stein. “This is a sign of progressive disease. We typically do tomography and difference maps six months to a year after LVC. This tells us how we accomplished the improvement in corneal regularity; it shows the areas we’ve steepened or flattened. The epithelium may not be totally mature at six months, and changes can occur even at one year.”
TG-PRK Limitations
Patients will get the most benefit from TG-PRK if the dioptric difference across their corneas is 10 D or less, says Dr. Stein. “If there’s marked irregularity of the cornea, it can be difficult to regularize because of the way the software works,” he explains. “If a patient has, for example, 10-D of difference across the cornea, the software would flatten the steep area by 5 D and steepen the flat area by 5 D, and that’s about the most the laser can do. Generally, less than a 10-D difference is an important threshold, as well as having enough cornea to work with—you want to have a thickness of at least 450 to 460 µm.”
If the dioptric difference is too great or the cornea is too thin for TG-PRK, one approach you can try is PTK, he says. “When the cornea is irregular, it’ll typically have an irregular epithelium,” says Dr. Stein. “Where the cornea is steepest, the epithelium will be thinner, and where the cornea is flatter, the epithelium will be thicker. By doing PTK of a constant amount, we end up removing a little more tissue over the steep part of the cornea, and that can reduce irregular astigmatism.”
 |
|
Figure 2. It’s important for patients to undergo annual tomography post-LVC to screen for ectasia, no matter what the UCVA is, experts say. Changes are likely to occur first in posterior elevation. This patient underwent LASIK in 2007 (left), but 2019 imaging (center and right) revealed ectasia development despite UCVA 20/20. The patient’s pachymetry map (center) and posterior elevation (right) are shown. |
Dr. Stein cautions that TG-PRK may worsen any corneal scarring already present. “Fortunately, most of the corneas we operate on are clear, so that isn’t an issue.”
One additional challenge with TG-PRK is the indirect nature of the correction. “We can’t directly steepen or flatten anything,” says Dr. Kershner. “If you want to steepen one area, you have to indirectly flatten an area 90 degrees away.”
“Topography-guided PRK isn’t perfect,” he continues. “Corneal behavior isn’t an exact one-to-one ratio when it comes to steepening and flattening. If you flatten one area by a given amount, in theory you’ve steepened the cornea 90 degrees away by the same amount, but it doesn’t always work out perfectly. You have to be very careful about balancing it, like a teeter-totter. It’s impossible to flatten one part without the other end coming up.”
He says this is where surgeons often run into trouble. “In the quest to eliminate astigmatism, ablating too much tissue to get the patient right where you want them can lead to an ectatic cornea. Now you’ve got a real problem on your hands. You don’t want to put a patient through a corneal transplant.
“It helps to know where the patient started in their refractive surgery journey,” he adds. “Now, we’re seeing the population who had excimer laser refractive surgery years ago who are now in the cataract age group, and they’re showing up for cataract surgery but we don’t know where their cornea started.
“If you’ve been taking care of a patient for years and have their early records, you can see what corneal topography, keratometry and wavefront data they’ve had,” he says. “This puts you in a better position to analyze your approach and know what you shouldn’t attempt. Many of the patients we’re seeing now had radial keratotomy, and we should be concerned about reopening old incisions and the like.”
Strategies for Success
Here are some tips for improving your irregular astigmatism patient’s visual acuity:
• Get to know the patient. “The key to success is getting to know your patient,” says Dr. Kershner. “I know that’s hard with a busy office and having to see many more patients than you used to, to make the same amount of money. It’s hard to take that time, but you need to—especially with a refractive surgery patient. If you don’t know their needs, you’ll get burned.”
He says discussing expectations, explaining the worst outcomes, and asking what the patient will be happy with are important to include in your counseling. “Always under-promise and over-deliver.”
• Stay alert for false ectasia patterns. These may result from measurement errors or previous decentered ablations. “If the patient fixates below the central axis of the videoscope, you’ll see a pattern of inferior steepening,” Dt. Stein says. “Be sure to check the pupil position relative to the center of the topographic map and repeat the imaging.”
He adds that a decentered hyperopic ablation is more likely to produce a pattern resembling ectasia. “A focal area of steepening on anterior curvature topography or anterior elevation tomography may be mistaken for ectasia,” he says. “Unlike keratoconus, however, you won’t see significant posterior elevation on tomography.”
• Never increase the power of the correction or shift its axis. “Patients can’t tolerate this,” says Dr. Kershner. “This is due to neuroadaptation. It’s a process ingrained in the brain to varying degrees. Younger people can neuroadapt more quickly to an alteration in optical input to the visual cortex, but the older you get, the more difficult it becomes.”
“If you change the patient’s axis, the magnifying effect of plus-cylinder astigmatism creates some degree of meridional optical perception differences,” he continues. “They can end up with a meridional anisometropia or meridional amblyopia. This is especially common in the genetic population of Native Americans. If you alter a patient’s optical apparatus to change their visual perception, the brain can’t neuroadapt to live with it.”
• Consider each case individually. “TG-PRK can also be used when irregular astigmatism is caused by lamellar keratoplasty, penetrating keratoplasty, arcuate relaxing incisions and corneal scores,” says Dr. Stein. “Every case should be considered on an individual basis in order to determine the risks and benefits of treatment.”
• Treat only the surfaces you can measure. “How you deal with the epithelium is important,” says Dr. Stojanovic. “Don’t remove it mechanically, because that will change the corneal optics completely. If we remove the epithelium, then the surface we’re treating isn’t the one we’re measuring, it’s the stroma. Transepithelial topography-guided PRK is the only way we can safely ablate irregular corneas at this point.”
Dr. Stojanovic says stromal surface topography-guided ablation is possible using epithelial thickness maps, but it’s not widespread yet. A case study from 2015 successfully used the treatment to significantly reduce a patient’s surgically-induced stromal surface irregularities and improve topography and visual quality.1
One thing to note with transepithelial ablation is the difference in ablation rate between the epithelium and the stroma. With good epithelial mapping, however, this is less of an issue. “There’s a slight difference in ablation rates, but we’re aware of it, and we know the average thickness of the epithelium when we’re removing it with PTK,” says Dr. Stein. “We like to use a large optical zone and transition down. Typically the optical zone is about 7 mm with the PTK, with a blend out to 9 or 9.5 mm. If we remove 50 µm with the Allegretto, we know we can induce a mild degree of myopia, say -0.75 D. We can account for this when trying to give patients the best uncorrected vision.”
• Be aware of the posterior cornea’s influence. “We can’t treat the posterior cornea, so if the irregular astigmatism is mainly due to posterior corneal irregularities, it’s better not to treat that patient because they won’t get better,” says Dr. Stojanovic. “It’s inaccessible to us, at this point. Wavefront-guided PRK would take the posterior cornea into account, but this approach isn’t usually good enough to handle irregular corneas.”
New Treatment Modalities
Dr. Stojanovic says that emerging modalities for irregular corneal optics are taking into account the posterior cornea and its influence on total corneal optics. “In the future, we’ll have total corneal ray-tracing-guided ablation for very customized care,” he says. “Alcon has released a ray tracing system, but right now it doesn’t separate corneal ray tracing from total-eye ray tracing. Oculus’ Pentacam AXL Wave also has total-eye ray tracing.”
Ray tracing optimizes refractive surfaces in the eye until the measured wavefront equals the simulated ideal wavefront for a particular patient. It does this by calculating an ablation profile for a laser using data from several measurements, including anterior and posterior corneal topography and the crystalline lens. While wavefront also considers the entire eye’s optical properties, it’s based on approximation, and its calculations are derived from a single measurement method, making it inherently less precise and less ideal for true customized treatment, say experts.2
Drs. Stojanovic, Stein and Kershner have no related financial disclosures.
1. Reinstein DZ, Gobbe M, Archer TJ, et al. Stromal surface topography-guided custom ablation as a repair tool for corneal irregular astigmatism. J Refract Surg 2015;31:1:54-59.
2. Schumacher S, Seiler T, Cummings A, et al. Optical ray tracing-guided laser in situ keratomileusis for moderate to high myopic astigmatism. J Cataract Refract Surg 2021;38:1:28-34.