Kenneth J. Mandell, MD, PhD, Aron Shapiro and Amanda J. Howe
Andover, Mass.
Varicella zoster virus, also classified as human herpes virus 3, is the etiologic cause of two very common diseases, varicella (chickenpox), which affects more than 90 percent of the population by adolescence (and almost 100 percent by age 60); and herpes zoster (shingles) which is experienced by about 30 percent of the population and becomes increasingly virulent as patients age. The United States experiences over a million herpes zoster cases each year.1,2 VZV has been studied for years, beginning in 1875 when the infectious nature of the attendant diseases was first revealed.3 In 1943, researchers hypothesized that shingles could be due to a reactivation of an earlier exposure.3 Then, in 1986, investigators decoded the VZV genome,4 leading to a varicella vaccine in 1995 and, more recently, a zoster vaccine in 2007.5 But the vaccine has not received the wide acceptance everyone anticipated, even though the virus is of considerable concern for many doctors.
In this month's article, we'll take a look at the herpes virus and its vaccine, and how ophthalmologists can play an important role in protecting patients from the associated disease.
Virology and Immunity
Like herpes simplex virus, varicella zoster virus grows and spreads rapidly, and has the ability to infect and destroy a number of host cells. Furthermore, following initial infection, VZV lies latent in the ganglionic tissue, from which it can awaken and cause illness years to decades later.1
The greatest risk factor for herpes zoster is immune status which, incidentally, deteriorates with age. Immunity to VZV is dependent upon primary infection or exposure at a young age and subsequent, periodic re-exposure to the virus.1 This often occurs through contact with children during varicella outbreaks, subclinical reactivation of endogenous latent virus, or a combination. During re-exposure to the virus, helper T cells carry immunologic memory and stimulate B cells to synthesize antibody at any sign of VZV provocation, thereby "teaching" new generations of leukocytes to recognize the virus and arm with the appropriate de--fense. Thus, each subsequent exposure acts as a sort of "booster," delaying HZ outbreak for decades.1 As people age, the ability of their immune system to suppress latent VZV virus gradually diminishes and, as a result, the risk of reactivation of latent VZV increases.
Clinical Course
Classic herpes zoster presents as an acute, often painful, vesicular eruption and is associated with fever, malaise and headache. Occasionally, it may be associated with transient mood changes, depression and insomnia.6 The prevalence of HZ does not differ by gender, nor is there any seasonal association with the disease. As mentioned above, the underlying immune status of the patient is, however, the most important factor.
Patients who are immunocompromised, whether due to HIV or immunosuppressive drugs, are at much greater risk for developing HZ and having a prolonged course of disease.6 As HZ is more typical in the elderly population, younger patients who present with symptoms should be tested for an underlying immune disease.
About half of immunocompetent adults develop complications of HZ. The most serious complication is herpes zoster ophthalmicus, which occurs in 10 to 20 percent of pa-tients with HZ.6 HZO is characterized by a unilateral skin rash in the distribution of the first branch of the trigeminal nerve. It can present with or without vesicles. When HZO occurs without vesicles, the telltale symptoms are pain, an odd sensation of hypoesthesia along the ophthalmic branch of the fifth cranial nerve (5-1) and a decrease in corneal sensation. At first, patients may have hypersensitivity due to in--flamed nerves but then they regress to hyposensitivity.
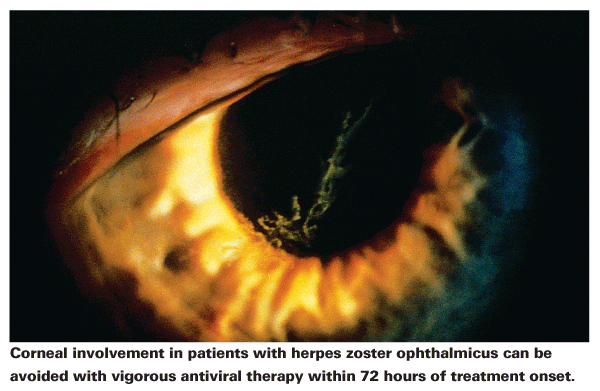
Even decades after HZO infection, the eye and adnexal tissue never return to their original state. One side of a patient's hairline tends to retain sensations of hypoesthesia, and patients live with effects such as decreased ocular sensation forever. Only herpes zoster, herpes simplex and leprosy cause a decrease in corneal sensation, so this and hypoesthesia can be used to retrospectively diagnose previous HZO. Patients may also develop keratitis and/or ulcers, and vascularization and scarring can, rarely, lead to perforation. HZO patients are at risk for anterior chamber complications as well, including keratouveitis and keratic precipitates in addition to photophobia and dramatic ocular pain. All of these side effects can continue to haunt patients long after infection.
Early use of systemic steroids may impede development by thwarting scarring, but post-herpetic neuralgia is another potential outcome. PHN is a painful condition affecting the nerves and skin, and is thought to arise as a result of the virus's proclivity for residing in nerves. Thus, many people continue to experience nerve-associated pain even after the visible symptoms of HZ have subsided. Various topical treatments for PHN are also available, including neurontin, nortriptyline and topical lidocaine cream. Furthermore, although neurologists can prescribe anti-depressants as a palliative treatment for PHN, they are not as effective as early, aggressive treatment—which could actually negate the need for anti-depressants.
Patients who develop HZO should be referred to an ophthalmologist, as vigorous antiviral treatment can prevent corneal involvement if initiated within 72 hours of symptom onset. One study in immunocompetent pa--tients found that 63 percent developed inflammatory reactions in the affected eye, but that their final visual outcome was acceptable. Decreases in best-corrected visual acuity, affecting 23 percent of patients at one month, were only experienced by 10 percent of patients at a six-month follow-up.7 Functional vision may therefore be retained in HZO patients, provided they receive the proper treatment following diagnosis.
Antiviral Therapy
Antiviral therapy should be initiated at the first sign of HZO infection to avoid serious complications. The three oral antiviral agents approved for treatment of HZO in immunocompetent patients are acyclovir, famciclovir and valacyclovir. Evidence suggests that all three medications reduce viral shedding and lesion formation; reduce the time to rash healing and decrease the severity; and decrease the duration of pain associated with herpes zoster.8 These drugs are most effective within 72 hours of rash onset, but some benefit has been observed with initiation of therapy up to one week afterward.8 Despite demonstrating efficacy in the acute phase of the disease, however, these oral antiviral medications don't prevent a recurrence of HZO after the initial three-day window.1 For immunocompromised patients who require more intensive therapy, intravenous formulations are available.
Prevention
Experience with the varicella vaccine for chickenpox (Varivax, Merck) provides an interesting context in which to consider VZV. By the year 2000, vaccine coverage of this typically innocuous disease reached over two-thirds of 18- to 35-month-olds, and there has been no evidence of increasing disease rates in older children in the past decade. The program has been extremely successful. Hospitalizations due to varicella have declined 88 percent, and only 3 percent of vaccinated children experience breakthrough infections.6
However, the lower incidence of wild-type varicella outbreaks has had negative consequences for adults. Fewer exogenous exposures to the virus by adults living or coming into contact with children have increased the incidence of HZ among adults under 50 years of age, whose cell-mediated immunity (CMI) used to be "boosted" by these exposures.6 Therefore, the incidence of HZ in the elderly is expected to rise.
Merck's Zostavax garnered U.S. Food and Drug Administration approval following trials involving more than 38,000 adults. It is indicated for all immunocompetent persons aged 60 and older and the sooner the vaccination is administered, the better the chances of being protected against shingles.
The Shingles Prevention Study, which formed the basis of the FDA approval, was organized by the Veteran's Administration.9 Subjects were followed for a mean of 3.13 years after vaccination, and the incidence of HZ was recorded during this time.
In the study, 937 cases of HZ were documented, meaning that vaccine efficacy for reducing HZ was about 51 percent (p<0.001), with a 67-percent reduction in PHN.9 The reduction in the incidence of HZ was significantly greater in participants aged 60 to 69 years (63.9 percent) versus those older than 70 years (37.6 percent).9
Furthermore, global epidemiological data suggest that the ideal age for HZ vaccination may be closer to 50 to 55 years,1,10 though further studies must be performed to verify this hypothesis.
Zostavax and Your Practice
Medicare Part D covers some of the cost of the Zostavax vaccine, and private insurance companies vary in their coverage. The Centers for Disease Control and Prevention approximates the cost of the vaccine to be about $150.2 Despite its relatively low cost and widespread availability, as of 2007 only 2 percent of the eligible population (persons over age 60) had re-ceived the vaccine.
Surveys were conducted among patients eligible for the vaccine to investigate the reason for such low vaccination rates. Surprisingly, a full 73 percent of respondents had not even heard of the vaccine, though 78 percent said they would get vaccinated if their doctor recommended that they do so.1 These results suggest that there's an opportunity for physicians, especially ophthalmologists, to educate patients about this option and encourage their participation in vaccination programs.
Ophthalmologists regularly provide care to the aging population at risk for developing herpes zoster ophthalmicus, and are well aware of the potential severity of the disease.
Ophthalmologists are therefore in an excellent position to administer this vaccine to eligible patients who are at high risk of developing HZO. The vaccine is relatively safe and the contraindications are clearly defined. Elderly patients who are in good health and not taking immunosuppressive medications would likely benefit from the VZV vaccine, and ophthalmologists have a unique opportunity to provide this option to them.
When one considers the relative frequency of HZO and its potential for debilitating pain and dramatic decrease in vision, vaccination is a worthwhile consideration for ophthalmologists.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Liesgang TL. Varicella zoster virus vaccines: Effective, but concerns linger. Can J Ophth 2009;44:4:379-84.
2. ACR. Herpes zoster (shingles) vaccine guidelines for immunosuppressed
3. Garland J. Varicella following exposure to herpes zoster. N Engl J Med 1943;228:336-7.
4. Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol 1986;67:1759-816.
5. Liesgang TL. Varicella-zoster virus eye disease. Cornea 1999;18:5:511-31.
6. Liesgang TL. Herpes zoster ophthalmicus: Natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2007;115:2:S3-12.
7. Zaal MJW, Volker-Dieben HJ, D'Amaro J. Visual prognosis in immunocompetent patients with herpes zoster ophthalmicus. Acta Ophth Scan 2003;81:216-20.
8. Volpi A. Severe complications of herpes zoster. Herpes 2007;14(Supplement 2):35-9A.
9. Gnann JW. Vaccination to prevent herpes zoster in older adults. J Pain 2008;9:1:S32-6.
10. Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Ger Soc 2007;55:1499-507.



