The future for synthetic corneal inlays appears bleak. According to John Hovanesian, MD, who practices in Laguna Hills, California, it’s clear that widespread market adoption of corneal inlays has not yet happened. “There were good companies and, in the case of Kamra, good products, but there has not been widespread acceptance,” he says.
He adds that there are several barriers to patient adoption of corneal inlays. “As we know from experience, the two biggest barriers to LASIK are fear and cost,” Dr. Hovanesian continues. “The same is true for corneal inlays. In the case of fear, I don’t think patients are much more scared than they would be of LASIK, but most people don’t know anyone who has a corneal inlay. Additionally, it’s a device that requires patients to adapt to some degree of monovision, because it’s only in one eye. That causes patient uncertainty about how well it will work for them. Most people who have not tried monovision have a negative reaction to the idea when you present it. In addition, the cost of a Kamra inlay, although it’s in only one eye, is greater than the cost of LASIK. In some cases, the patient may have hyperopia or astigmatism, so he or she may have to undergo a refractive procedure as well. So, we have to overcome both fear and cost concerns with corneal inlays.”
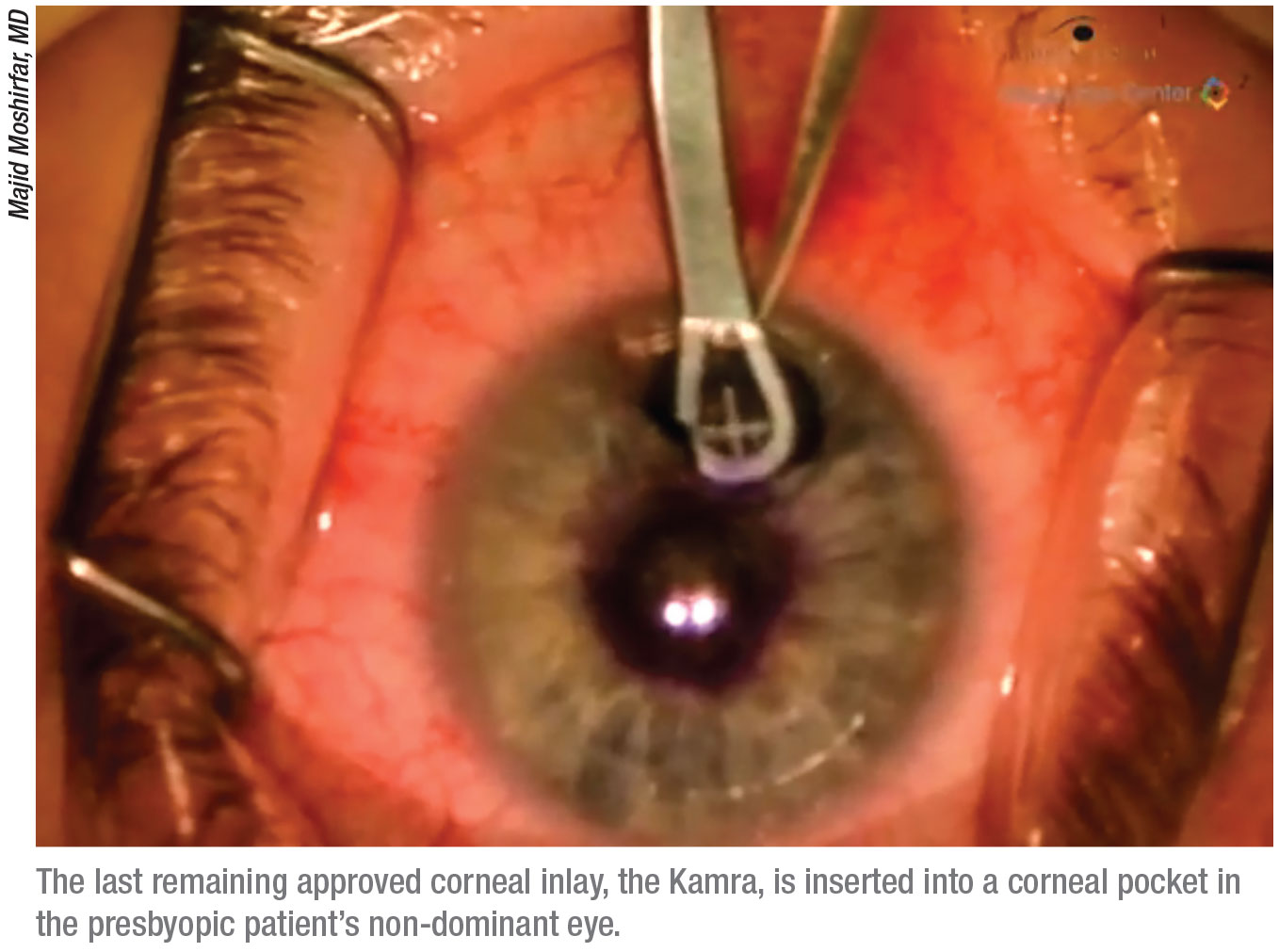
Only One Remains
Several corneal inlays have come to market in recent years, but the
Kamra corneal inlay is the only one still available. Though the inlay’s adoption rate by surgeons isn’t anything like when PRK or LASIK were first approved, Dr. Hovanesian believes that Kamra will stick around because the company is set up to support it. “If the whole company’s existence depended upon just Kamra, it would be hard to keep a company going on a small product like that,” he says. “But CorneaGen has a wide variety of products that appeal to cornea specialists, and they don’t need
Kamra to be a blockbuster product for them. I don’t think the company has any grand designs that it’s going to take over the world of presbyopia correction, but I believe it’s a valuable offering they will continue to make available.”
 |
Richard Lindstrom, MD, who is in practice in Minneapolis, notes that another corneal inlay has been taken off the market: the Raindrop from ReVision Optics. It’s a hydrogel that was placed under a flap to cause a central steepening of the cornea and basically create a multifocal cornea. “That particular product was removed from the market because it had some adverse outcomes, primarily related to interface haze between the hydrogel and the cornea,” Dr. Lindstrom says. “Additionally, it had decentration issues. And so, while it actually did achieve FDA approval, it was pulled off the market.”
Dr. Hovanesian adds that, with ReVision Optics, both the company and the technology ultimately failed, despite a respected team and a promising product. “We think the problem was the material,” he says. The hydrogel material was probably not as biocompatible as another material might have been, though many patients did well. In my practice, I did about 30 of the Raindrop implants, and the vast majority of my patients did very well, but not everyone in the trial did. In the end, the FDA issued a warning saying that no more of these should be implanted. And, in fact, we’ve removed some of them just to prevent future problems with the implants.” (The CyPass glaucoma implant from Alcon suffered the same fate. Some late complications showed up after Food and Drug Administration approval, so it was taken off the market.)
Dr. Lindstrom says the Raindrop’s failure may have impacted other products. “The failure of this inlay in the marketplace obviously created a meaningful ripple effect, as far as concerns, and also created a negative perception about corneal inlays in general for the treatment of presbyopia,” Dr. Lindstrom says. He notes that the Presbia company was developing a small-diameter intracorneal lens that increased refractive index and was placed in a deep pocket in the cornea. “Basically, the failure of Raindrop limited Presbia’s ability to raise the capital needed to pursue its business plan,” Dr. Lindstrom says. “The company still exists, but it’s not active at this time. Again, I think there was a bit of a negative ripple from the failure of Raindrop, which impacted their ability to raise money.”
San Diego surgeon Michael Gordon was involved in the Presbia study and says the device achieved good results. “We had very few issues with biocompatibility, but it does occur,” he notes. “These issues respond very well to steroids. But we have newer IOLs and a different age population. For the 40-year-old presbyope, I think we’re better off doing laser monovision. Lasers are so good now that you achieve the results you want without having to insert a foreign material into the eye.”
The Kamra inlay was initially brought to the marketplace by AcuFocus, which eventually sold the inlay to SightLife Surgical/CorneaGen. “The market for the inlay is small for several reasons,” says Dr. Lindstrom. “First, the presbyope, particularly the emmetropic presbyope, is highly risk-averse. Corneal inlays are usually used to treat mild to moderate presbyopia. These patients have perfect distance vision and often good intermediate vision, so very few patients are willing to accept the risk of a surgical procedure for the treatment of their presbyopia. We now better understand the market. While there are 120 million presbyopes in the United States, only a small number of them are ready to undergo surgery.”
Another reason that corneal inlays have struggled is that they’re in competition with monovision. “Monovision is, of course, stiff competition, so many of the patients who might have been interested in the intracorneal lens choose monovision instead,” Dr. Lindstrom says. “The third reason that the inlay market is small is the negative ripple effect of seeing late complications occur in ReVision Optics’ Raindrop, and concerns that these same issues could occur in other intracorneal lenses as well. To date, it hasn’t been seen as frequently as with the Kamra inlay, but it has definitely dampened enthusiasm, I believe, for the whole concept of placing a synthetic inlay into the cornea for the treatment of presbyopia.”
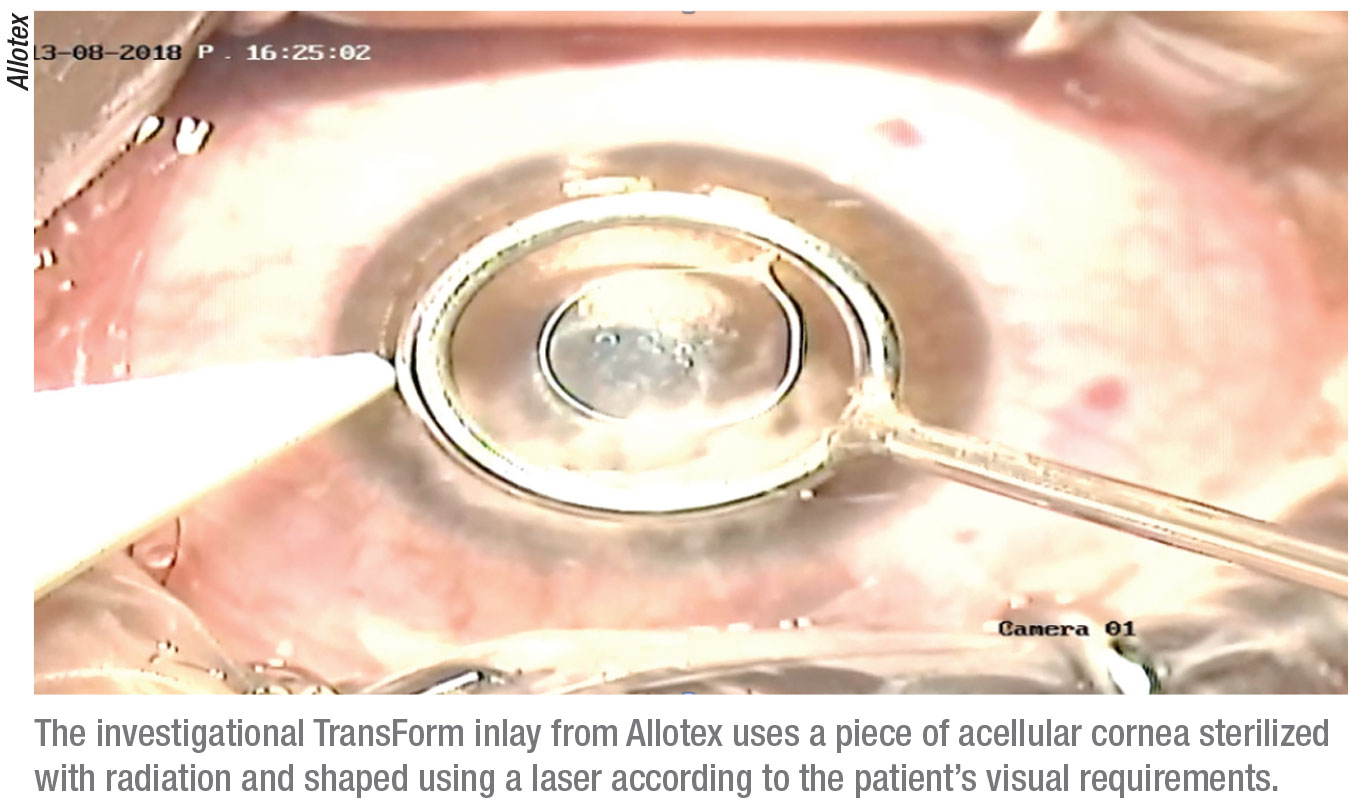
One company still working in this field is Allotex. Its product is similar to the Raindrop, but instead of using a synthetic material, it’s using human corneal tissue. “The human tissue negates the concerns about interface haze,” says Dr. Lindstrom. “They are currently pursuing an early clinical trial outside of the United States.”
The Allotex TransForm lenticule is a piece of acellular cornea that’s sterilized with electron beam radiation and then shaped using a laser.1 The goal is to enhance the visual performance of the patient with a material that’s 100-percent biocompatible and precisely shaped for the patient’s needs. It’s indicated for intrastromal implantation to provide near vision in the non-dominant eye of patients 41 to 65 years old with presbyopia and a manifest refraction spherical equivalent of +1 D to
-0.75 D with less than 0.75 D of refractive cylinder, who don’t require any type of correction for clear distance vision, but who do require near correction of +1.75 D to +3.50 D of reading add.
The Future
Dr. Lindstrom is interested to see results with the Allotex inlay. “I think that will be of interest because of the obvious biocompatibility,” he says. “I think there will continue to be a small following for the Kamra inlay because it does work well. I don’t see that inlay failing because of complications, but it won’t be a meaningful product as far as number of inlays placed. And, there is some interesting competition coming. Probably the most exciting thing coming is the pharmacologic treatment of presbyopia with eye drops.”
 |
According to Dr. Lindstrom, there are two approaches to this treatment. One is to uncross-link the human lens and increase its elasticity, which is being pursued by Novartis. Additionally, three companies, Presbyopia Therapeutics, Orasis and Allergan, are studying miotic drops. “These drops make your pupils small to basically induce small aperture optic outcomes, much like the Kamra inlay does with surgery,” Dr. Lindstrom says. “These are the same drops that have been used for decades for glaucoma, so they have a long track record of safety and they are reversible. For example, if someone just wanted to see better during the day while at work but is happy to wear readers at night, he or she can use one to two drops during the workday, or if he or she is going out at night on a date and wants to read the menu without readers, he or she might use a drop just in the evening. And, depending on the drop, some might last two to four hours, while others might last six to eight hours, but they would basically be creating temporary improvement in near vision with an eye drop. Those companies’ drops are all in clinical trials.”
These drops have shown promise for the temporary treatment of presbyopia in trials. A study conducted in Egypt found that carbachol plus brimonidine seems to be an acceptable, safe alternative to corrective lenses and surgical procedures.2 The prospective, double-masked, randomized, placebo-controlled clinical trial included 48 patients who were naturally emmetropic and presbyopic. All patients were between the ages of 43 and 56 years with an uncorrected distance visual acuity of at least 20/20 in both eyes without additional ocular pathology. The 30 eyes in the treatment group received a single dose of 2.25% carbachol plus 0.2% brimonidine eye drops. The control group (18 eyes) received placebo drops. Drops were given in a masked fashion in patients’ nondominant eyes. Their pupil size and both near and distance visual acuities were evaluated before and after treatment at one, two, four, eight and 10 hours by a masked examiner.
The investigators reported statistically significant improvement in near visual acuity in all patients who received carbachol plus brimonidine drops. All patients in the study said they liked and would use the therapy if it was available.
Dr. Hovanesian adds that he has heard a lot of feedback about how much patients like the idea of eye drops. “Then, it becomes a question of how tolerable and expensive the drop is,” he says. “But I think there will be wide acceptance of the drops.”
Additionally, a couple of surgical procedures, LaserACE and VisAbility microinserts, can be performed on the sclera to enhance accommodation, either by expanding, indenting or weakening the sclera. “These are in clinical trials, but they are both surgical procedures with some invasiveness and morbidity,” Dr. Lindstrom notes.
“Currently, I’m most excited about the miotic drops that can either treat presbyopia at the source by increasing natural lens elasticity or transiently improve near vision using small diameter aperture optics,” Dr. Lindstrom says. “So, I think that’s probably going to be the winner among the options I see coming along now. They’ll probably be available in another year or two in the United States, and maybe before that outside of the United States.”
| “Currently, I am most excited by the miotic drops that can either treat presbyopia at the source by increasing natural lens elasticity or transiently improve near vision using small-aperture optics.” — Richard |
Dr. Hovanesian adds that treating presbyopia is tough. “Objective data aren’t always so objective,” he says. “Reading vision and overcoming presbyopia are somewhat effort-dependent. When reading on the chart, it’s not like there’s as crisp an endpoint as there is when we’re looking at a trial for macular degeneration or some other measure of visual acuity. With presbyopia, patient effort can influence the outcome, and that means that the endpoints in these studies are a little bit softer than normal visual acuity endpoints. So, a company can have really good-looking data, but the product may not work quite as well as you’d think,” he cautions. REVIEW
Dr. Lindstrom has a financial interest in CorneaGen and Orasis. Dr. Gordon has a financial interest in Presbia. Dr. Hovanesian is an equity owner of CorneaGen, and was a clinical investigator for the Raindrop corneal inlay.
1. TransForm product description. allotex.com.
2. Abdelkader A. Improved presbyopic vision with miotics. Eye Contact Lens 2015;41:5:323-327.



