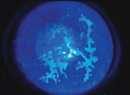
When and why endophthalmitis will rear its ugly head is a mystery. Although we know much about it, it remains challenging to consistently prevent and treat, and the growing problem of bacterial resistance makes it even tougher. Even though this infection is relatively rare, with vision loss as its potential outcome we can't afford to underestimate it. This column will review our current knowledge of the elusive problem of endophthalmitis.
Endogenous & Exogenous
Endophthalmitis is characterized by inflammation of the anterior and posterior segments of the eye, typically secondary to bacterial or fungal infection. In general, it's thought of as a rare complication of intraocular surgery; however, this doesn't paint the entire picture. Most endophthalmitis cases are indeed exogenous in nature, arising primarily from intraocular surgery (62 percent of cases), penetrating trauma (20 percent) and filtering blebs (10 percent). Only 2 to 8 percent of infection cases arise from endogenous origins.1,2 Endogenous, or metastatic, endophthalmitis occurs when a primary site of infection elsewhere in the body allows for the migration of the infectious organism to the eye.
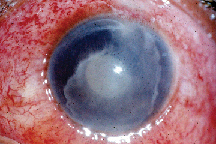 |
| In severe cases, endophthalmitis can cause corneal opacification and infiltrates, as well as a fever. |
Causative Organisms
The profile of causative microbes differs for each type of endophthalmitis, though they're often those normally found nearby, such as on the conjunctiva. For this reason, conditions such as blepharitis and/or nasolacrimal duct infections/obstructions can be risk factors.2
From 56 to 90 percent of organisms causing infection in postop endophthalmitis are gram positive, the most common of which are S. epidermis, S. aureus, and streptococcus. Gram-negative bacteria are responsible for 7 to 29 percent of cases, most commonly various species of proteus, P. aeruginosa, and haemophilus. Some of these organisms are more closely associated with certain procedures than others, for example bacillus is associated with penetrating trauma and haemophilus with late bleb-associated endophthalmitis.2
The most common post-trauma infections, in descending order, include gram-positive organisms such as S. epidermis, streptococcus and bacillus, followed by gram-negative organisms, and finally fungi.3,4,5 Like postop endophthalmitis, there is typically only one species present.
Cases of endogenous endophthalmitis present a somewhat different profile. With bacterial infection, gram positive is, as above, more common than gram negative. However, fungal infection with Candida is the most common causative organism in cases of endogenous endophthalmitis.1,2 One retrospective study of culture-proven endogenous endophthalmitis cases found that 62 percent were fungal, 33 percent gram positive, and 5 percent gram negative.6
Another retrospective study evaluated 278 patients, including all sub-categories of endophthalmitis, and most commonly yielded S. epidermidis (27.8 percent). Gram-positive organisms comprised 78.5 percent of those identified, gram negative 11.8 percent, and fungal 8.6 percent.7
Signs, Symptoms & Cultures
Exogenous endophthalmitis is usually seen in patients who have undergone intraocular surgery. It typically presents with increasing ocular pain or discomfort and a decrease in visual acuity. These changes are usually accompanied or followed shortly by complaints of lid swelling, conjunctival hyperemia, photophobia or discharge. One study of 854 patients found blurred vision to be the most prevalent presenting sign, with ocular pain and hypopyon present in 75 percent of patients.8 Additional signs include corneal edema and membrane formation on an intraocular lens, and, in severe cases, corneal opacification and infiltrates and a fever.
One of the keys in identifying endophthalmitis is being aware of the patient's ocular surgery, trauma and systemic medical history. It's also important to consider endophthalmitis even if there's no recent history of eye surgery. Though approximately a quarter of postop endophthalmitis cases present within three days of surgery and at least 80 percent are evident within a week,9 there are documented cases of endophthalmitis that occurred weeks, months or even years postop. A retrospective analysis of S. pneumoniae endophthalmitis cases found that a third had a delayed onset, with the appearances ranging from three months to 10 years postop.10
The patient population for endogenous endophthalmitis can include any age, race or gender. A quarter of cases are bilateral. The manner of infection in endogenous endophthalmitis also makes it more likely in those patients with compromised immune systems.
Incidence: Is It on the Rise?
The incidence of exogenous postop endophthalmitis has been tracked in numerous large, usually retrospective, studies. In 1974, one study of 36,000 patients revealed an incidence of 0.086 percent; others found incidences between 0.051 to 0.3 percent, and 0.12 to 0.17 percent.2
More recent retrospective data has identified a postoperative endophthalmitis incidence of 0.14 percent,11 and even around 0.5 percent.12 In an unpublished study of 17,438 cataract surgeries performed in a four-year period, Halifax, Nova Scotia, researcher Bryce Ford, MD, found the incidence to be 0.206 percent.
The rate of infection in post-trauma cases is much higher: 3 to 17 percent.13 In rural areas the rate of post-trauma infection is even higher, approximately 30 percent.14
The evidence in both exogenous and endogenous endophthalmitis indicates that the rates of each are rising. Contributors to the rise in endogenous infection include the increases in the number of complex, invasive medical procedures being performed, as well as the lengthened duration of patient survival with immunosuppressed conditions. Both of these factors increase the potential for metastasis of infection via blood-borne pathogens.
As current epidemiological studies appear to suggest an increasing incidence of postop endophthalmitis, researchers are developing a heightened interest in evaluating the variables that may be contributing to this. Some research suggests that clear-corneal cataract surgery wounds can result in a risk of postop endophthalmitis three times higher than the risk associated with scleral-tunnel incisions.15 However, others have noted that the difference in endophthalmitis between these two incision types wasn't significantly different.16 In the endophthalmitis cases studied by Dr. Ford, a temporal, clear-corneal incision was used in 92 percent of them. Debates about other such topics, including incision location—temporal corneal vs. superior sclerocorneal—and differences in risk with various IOL polymers, are also under discussion in the literature.17,18 These are areas that warrant further, well-designed studies.
It's also possible that new macular degeneration treatments that involve intravitreal injection could contribute to the incidence of endophthalmitis. Safety evaluations from the Macugen trial show infection rates of 0.16 percent per injection and 1.3 percent per patient.(D'Amico DJ, et al. ARVO Abstract 2363, 2004) An exception to this is Retaane (anecortave acetate) which uses a juxtascleral depot instillation.
Treatment
Prophylaxis is, of course, the first step in managing postop endophthalmitis. Preventive measures can include the use of topical antibiotics pre- and postop, povidone-iodine antisepsis, saline irrigation, postop subconjunctival and intracameral antibiotics and perhaps even lash trimming.19,20 Although these measures may reduce bacteria found on or near the eye, they may also be allowing for overgrowth of resistant strains. In addition, this raises the question of whether the same antibiotics should be used both pre- and postoperatively.
Though lab culture of the bacteria causing the infection is usually an important step when endophthalmitis becomes evident, the often aggressive course of the infection requires the clinician to take action before culture results are available, making broad-spectrum antibiotics the most appealing choice. In fact, research has documented that changes in treatment selection are typically not made even if the bacterial identity is known.9
The standard therapy for postop endophthalmitis treatment is intravitreal injection with vancomycin and amikacin or ceftazidime to assure eradication of both gram-positive and gram-negative bacteria. Typically a single dose is sufficient, though some cases require repeat doses for virulent or slow-replicating organisms. In these cases, repeat dosing is possible, but retinal toxicity becomes a concern.2 Subconjunctival injection and topical therapy of similar drug combinations are often also added to achieve concentrations in the anterior chamber.
Additional therapeutic possibilities include corticosteroids, vitrectomy and systemic antibiotics. Corticosteroids' potent anti-inflammatory effects can help, though they must be weighed against their potential to impede an antibiotic. Vitrectomy is typically reserved for those cases that are at an advanced stage. One study found no additional benefit with vitrectomy as opposed to vitreous tap in patients with hand motion or better acuity, but noted three times the frequency of achieving improvement to 20/40 vision with the procedure in light-perception-only patients.9
Clinicians turn to systemic therapy more often in cases of fungal infection. Endogenous endophthalmitis can often benefit from systemic and/or intravenous antibiotics. These agents are considered of limited use in bacterial postop endophthalmitis, though, due to challenges in getting past the blood-eye barrier. Some newer antibiotics, however, are able to achieve improved penetration.2,20,21
A recent prospective study conducted throughout the U.K. provided an overview of the relative frequency with which each treatment is used, revealing that in managing postop endophthalmitis patients, intravitreal injection was administered in 96 percent of cases, oral antibiotics in 65 percent, subconjunctival in 30 percent, intravenous in 17 percent, and intravitreous steroids in 17 percent.11
Bacterial Resistance
Study of sensitivities of organisms causing endophthalmitis shows the greatest sensitivity of gram-positive bacteria is to vancomycin (100 percent), followed by gentamicin (78.4 percent), ciprofloxacin (68.3 percent), ceftazidime (63.6 percent), and cefazolin (66.8 percent).
The sensitivities for gram-negative bacteria were 94.2 percent for ciprofloxacin, 80.9 percent for amikacin, 80 percent for ceftazidime and 75 percent for gentamicin.7 There are concerns that prophylactic use of antibiotics such as vancomycin may be contributing to the development of resistant bacterial strains, though one study has concluded that ophthalmology's contribution to this is negligible.22 However, just because we're not the cause of the problem doesn't mean we will escape its effects.
The newest generation of fluoroquinolones appears to be the current weapon we need in the battle against bacterial resistance, though its benefits won't last forever.
Bacterial resistance, the history of antibiotics and our never-ending quest for the best treatment will be the topics of next month's column.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals.
Ms. Forbes is a research associate at Ophthalmic Research Associates.
1. Romero CF, Rai Mk, Lowder CY, et al. Endogenous endophthalmitis: Case report and brief review. Am Fam Physician 1999;60:2:510-4.
2. Ng EWM, D'Amico DJ. Postoperative endophthalmitis. In: Albert DM and Jakobiec FA, eds. Principles and Practices of Ophthalmology, 2nd Ed. Philadelphia: WB Saunders & Co., 2000:2441-2462.
3. Tran TP, Le TM, Bui HT, et al. Post-traumatic endophthalmitis after penetrating injury in Vietnam: Risk factors, microbiological aspect and visual outcome. Kin Monatsbl Augenheilkd 2003;220:7:481-5.
4. Abu el-Asrar AM, al Amro SA, al Mosallam AA, et al. Post-traumatic endophthalmitis: Causative organisms and visual outcome. Eur J Ophthalmol 1999;9:1:21-31.
5. Dieckert JP. Penetrating posterior segment trauma. In: Albert DM and Jakobiec FA, eds. Principles and Practices of Ophthalmology, 2nd Ed. Philadelphia: WB Saunders & Co., 2000:5222.
6. Scheidler V, Scott IU, Flynn HW. Culture-proven endogenous endophthalmitis: Clinical features and visual acuity outcomes. Am J Ophthalmol 2004;137:4:725-31.
7. Benz MS, Scott IU, Flynn HW, et al. Endophthalmitis isolates and antibiotic sensitivities: A 6-year review of culture proven cases. Am J Ophthalmol. 2004;137:1:38-42.
8. Wisniewski SR et al. Characteristics after cataract extraction or secondary lens implantation among patients screened for the EVS. Ophthalmology 2000;107:7:1274-82.
9. Vitrectomy Study Group: Results of the endophthalmitis vitrectomy study: A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 1995;113:1479.
10. Miller JJ, Scott IU, Flynn HW, et al. Endophthalmitis caused by Streptococcus pneumoniae. Am J Ophthalmol 2004;138:2:231-6.
11. Kamalarajah S, Silvestri G, Sharma N, et al. Surveillance of endophthalmitis following cataract surgery in the UK. Eye 2004;18:6:580-7.
12. Hanscom TA. Postoperative endophthalmitis. Clin Infect Dis. 2004;38:4: 542-6.
13. Callegan MC, Engelbert M, Parke DW, et al. Bacterial endophthalmitis: Epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev 2002;15:1:111-124.
14. Boldt HC, Pulido JS, Blodi CF, et al. Rural endophthalmitis. Ophthalmology 1989;96:12:1722.
15. Cooper BA, Holekamp NM, Bohigian G, Thompson PA. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol 2003;136:300-305.
16. Colleaux KM, Hamilton WK. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can J Ophthalmol 2000;35:373-8.
17. Meinkoff JA, Speaker MG, Marmor M, et al. A case-control study of risk factors for post operative endophthalmitis. Ophthalmology 1991;98:12:1761-1768.
18. Nagaki Y, Hyasaka S, Kadoi C, et al. Bacterial endophthalmitis after small-incision cataract surgery: Effect of incision placement and intraocular lens type. J Cataract Refract Surg 2003;29:1:20-6.
19. Olson RJ. Reducing the risk of postoperative endophthalmitis. Surv Ophthalmol 2004;49:2:S55-61.
20. Busbee BG. Advances in knowledge and treatment: An update on endophthalmitis. Curr Opin Ophthalmol 2004;15:232-237.
21. Mamalis N, Kearsley L, Brinton E. Postoperative endophthalmitis. Curr Opin Ophthalmol 2002;15:14-16.
22. Gordon YJ. Vancomycin prophylaxis and emerging resistance: Are ophthalmologists the villains? The heroes? Am J Ophthalmol 2001;131:3: 371-6.