Anti-VEGF Therapy
When treating branch or central retinal vein occlusion, anti-VEGF medications remain the gold standard. “The first line of treatment is still anti-VEGF medications. Avastin (bevacizumab), Lucentis (ranibizumab) and Eylea (aflibercept intravitreal injection) have all been used to successfully treat retinal vein occlusions. Corticosteroids are also effective. We have used triamcinolone and, more recently and more effectively, Ozurdex (dexamethasone intravitreal implant),” says Michael Singer, MD, who is in practice in San Antonio.
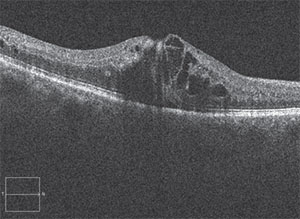 |
| This patient presented with the hallmark signs of branch retinal vein occlusion. |
To determine the efficacy of anti-VEGF injection, a recent retrospective study included 81 patients with retinal vein occlusion and macular edema who were naïve to anti-VEGF therapy.1 The researchers treated 26 eyes with ranibizumab and 33 eyes with bevacizumab. They also treated 22 eyes with bevacizumab and then switched them to ranibizumab. The main outcome measure was the change in acuity at three months, six months and at the final visit.
In the ranibizumab and bevacizumab groups, the mean visual acuity improved from 20/80 to 20/40 and from 20/125 to 20/60, respectively. The mean change in central subfield thickness was -186 µm for ranibizumab and -212 µm for bevacizumab. The mean time between injections was 94 ±21.1 days in the ranibizumab group and 103.8 ±10.5 days in the bevacizumab group. In the group that switched from bevacizumab to ranibizumab, mean initial visual acuity was 20/125. Visual acuity reached 20/60 at crossover and remained at 20/60 through the remainder of the study.
“However, the longer we have been treating retinal vein occlusion patients with anti-VEGF, the more we have realized that many of these patients can’t get off treatment and require injections anywhere from every four to six weeks to every three months,” says David Boyer, MD, who is in practice in Los Angeles. It appears that a true unmet need exists with anti-VEGF monotherapy.
Steroids Step In
Studies have shown that corticosteroids are also a safe and effective treatment option. The Standard of Care vs. Corticosteroid for Retinal Vein Occlusion Study showed that intravitreal triamcinolone acetonide effectively reduces macular edema and improves visual acuity in patients with RVO.2 Investigators conducted a secondary analysis of the incidence, risk factors and timing of IOP elevation occurring after IVTA, which can provide guidance for clinical decision-making and management of patients treated with IVTA. The study included 682 patients with macular edema secondary to retinal vein occlusion. Study participants were randomized to standard of care, 1 mg of IVTA, or 4 mg of IVTA therapy and were followed for a mean of 24.7 months. Kaplan-Meier incidences of IOP elevation greater than 10 mmHg from baseline at 36 months were 0.02, 0.09 and 0.45 in the standard of care, 1-mg IVTA and 4-mg IVTA groups, respectively. The 4-mg IVTA group also experienced higher rates of IOP-related events compared with the other groups. In patients treated with 1 mg and 4 mg of IVTA, the median number of days from time of first injection to IOP elevation greater than 10 mmHg from baseline was 34 and 52.5 days, respectively. The investigators concluded that the risk factors for an IOP-related event include a higher treatment dose, younger age and higher baseline IOP. The researchers added that IOP-related events may occur several months after the first IVTA injection, and physicians should consider these risk factors when assessing the risks and benefits of IVTA therapy and the need for long-term follow-up of patients who are at risk for this complication.
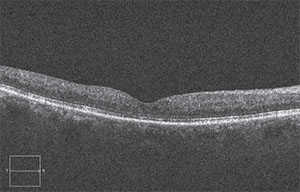 |
| After a treatment with anti-VEGF and eventually Ozurdex, the edema was greatly reduced and vision had improved. |
The dexamethasone implant (Ozurdex 0.7 mg, Allergan) has also been shown to effectively treat vein occlusion. A recent study evaluated the safety and efficacy of one or two treatments using dexamethasone intravitreal implants over 12 months in eyes with macular edema related to BRVO or CRVO.3
This study included 1,256 patients with vision loss caused by macular edema associated with retinal vein occlusion. At baseline, 421 patients received a dexamethasone 0.7-mg implant, 412 received a dexamethasone 0.35-mg implant, and 423 received a sham implant. At day 180, patients could receive a dexamethasone 0.7-mg implant if their best-corrected visual acuity was less than 84 letters or if their retinal thickness was greater than 250 μm, and this implant was received by 997 patients. Except for cataract, the incidence of ocular adverse events was similar in patients who received their first or second dexamethasone implant. Over 12 months, cataract progression occurred in 90 of 302 phakic eyes (29.8 percent) that received two dexamethasone 0.7-mg implant injections, compared with five of 88 sham-treated phakic eyes (5.7 percent). Cataract surgery was performed in four of the 302 (1.3 percent) phakic eyes that received two implants and one of 88 (1.1 percent) eyes that received the sham.
In terms of IOP effects, in the group receiving two 0.7-mg DEX implants, researchers reported an increase in IOP of 10 mmHg or greater from baseline in 12.6 percent of patients after the first treatment, and 15.4 percent after the second. The investigators found that the IOP increases were usually transient and controlled with medication or observation. After the second treatment, an additional 10.3 percent of patients initiated IOP-lowering medications.
An improvement in best-corrected visual acuity of 15 letters or more from baseline was achieved by 30 percent and 32 percent of patients 60 days after the first and second dexamethasone implant, respectively.
“Ozurdex works remarkably well in some patients. It is able to stabilize vision for longer periods of time, and in some cases, it can reduce the number of treatments that are necessary,” Dr. Boyer adds.
A Combination Approach
While anti-VEGF and corticosteroid treatments work well alone, there’s evidence that they work even better in combination, and according to Chicago-based vitreoretinal surgeon Seenu Hariprasad, MD, the treatment paradigm is headed toward combination therapy. “There is really good evidence in the literature that vein occlusion is a multifactorial disease. Of all the retinal diseases that we treat, branch retinal vein occlusion and central retinal vein occlusion cause the highest level of VEGF in the eye. There is also very good evidence that there is an inflammatory component that anti-VEGF monotherapy does not address. This may contribute to the high treatment burden with anti-VEGF therapy, so I am a big believer in incorporating other modalities of treatment, such as Ozurdex. I think a combination of an anti-VEGF and Ozurdex can be very powerful,” adds Dr. Hariprasad.
A 2014 study found that bevacizumab combined with dexamethasone implants produced greater improvements in macular thickness than bevacizumab therapy alone and required fewer bevacizumab injections in both BRVO and CRVO.4
The study included 30 eyes that were randomly assigned to receive either combination therapy or monotherapy with bevacizumab. All patients received intravitreal bevacizumab at baseline, followed a week later by dexamethasone implants or sham injections. Monthly bevacizumab injections were given if the central subfield thickness was less than 250 µm, and the combined group received a second implant after four or five months if the central subfield thickness was less than 250 µm.
At six months, patients receiving combined therapy required fewer bevacizumab re-injections compared to those receiving monotherapy (two compared to three). They also experienced greater mean reductions in central subfield thickness (-56 µm compared to +45 µm) and were more likely to have resolved all edema, which was considered to be a central subfield thickness less than 250 µm (seven of 11 eyes compared to two of 14 eyes). Mean visual acuity changes from baseline were similar between groups.
Dr. Hariprasad notes that imaging is critical in this disease. “Using OCT is one of the best ways to track treatment response,” he says. “We get a baseline OCT, and then we can determine whether the pharmacologic agent is working or not. So, it is a great way to track and quantify treatment. We also use fluorescein angiography to determine the amount of macular and peripheral ischemia and address both accordingly. I would recommend ultra-wide-field fluorescein angiography and OCT testing.”
According to Dr. Hariprasad, a potentially important advance in the treatment of retinal vein occlusion is CLS-1003 (Clearside Biomedical), which consists of a microneedle injection of the company’s Zuprata (triamcinolone acetonide) into the suprachoroidal space in combination with an intravitreal injection of Eylea. The therapy was developed for the treatment of macular edema associated with retinal vein occlusion.
In April, Clearside completed a small Phase II trial of 46 patients.5 In this study, patients who received concomitant administration of Zuprata and Eylea qualified for approximately 60 percent fewer intravitreal Eylea treatments than those patients who received Eylea alone. This is the first controlled, masked, randomized trial conducted in patients with retinal vein occlusion where the drug was administered through the suprachoroidal space. No serious adverse events were reported during the trial, and treatment was generally well-tolerated. “In the past, we just used an anti-VEGF,” Dr. Hariprasad says, commenting on the results. “Then, the corticosteroids came along and enhanced the way we treat the disease.”
According to Daniel H. White, CEO and president of Clearside, “Based on these results, Clearside intends to follow a 505(b)(2) NDA regulatory approval pathway and expedite the preparations for a Zuprata Phase III registration program for the treatment of macular edema associated with RVO.” REVIEW
Dr. Singer is a consultant for Allergan, Genentech and Regeneron. Dr. Boyer is a consultant for Allergan, Genentech, Roche, Bayer, Novartis and Regeneron. Dr. Hariprasad is a consultant for Alcon, Allergan, Bayer, Alimera Sciences, Clearside Biomedical, Janssen, Ocular Therapeutix, OD-OS, Optos, Regeneron and Spark.
1. Yuan A, Ahmad BU, Xu D, Singh RP, Kaiser PK, Martin DF, Sears JE, Schachat AP, Ehlers JP. Comparison of intravitreal ranibizumab and bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Int J Ophthalmol 2014;7:1:86-91.
2. Aref AA, Scott IU, Oden NL, et al; SCORE Study Investigator Group. Incidence, risk factors, and timing of elevated intraocular pressure after intravitreal triamcinolone acetonide injection for macular edema secondary to retinal vein occlusion: SCORE study report 15. JAMA Ophthalmol 2015;133:9:1022-1029.
3. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion: Twelve-month study results. Ophthalmology 2011;118:12:2453-2460.
4. Maturi RK, Chen V, Raghinaru D, Bleau L, Stewart MW. A 6-month, subject-masked, randomized controlled study to assess efficacy of dexamethasone as an adjunct to bevacizumab compared with bevacizumab alone in the treatment of patients with macular edema due to central or branch retinal vein occlusion. Clin Ophthalmol 2014;8:1057-1064.
5. Data on file, Clearside Biomedical. http://ir.clearsidebio.com/phoenix.zhtml?c=253669&p=irol-newsArticle&ID=2162115.
Suggested Reading:
1. Hariprasad S. Management of Retinal Vein Occlusion: Current Concepts. 2014. Slack Incorporated. https://www.amazon.com/Management-Retinal-Vein-Occlusion-Concepts/dp/1617116165/ref=sr_1_1?ie=UTF8&qid=1465932449&sr=8-1&keywords=hariprasad+retinal



