Surgeons' options with regard to intraocular lens design and material are many, and they're increasing. Variables include edge profiles, material and design styles. However, many ophthalmologists find it hard to determine if these variables actually make a difference in the real world, clinical setting.
"Lens design absolutely does impact patient outcomes," says Robert M. Kershner, MD, of Eye Laser Consulting in Boston, one of the world's foremost experts on new technology IOL optics. "I have studied lens design extensively for the last 25 years and there is no question that lens haptic, lens optics and lens edge design are critical for optimum performance of the IOL."
The clarity of the lens capsule is a key determinant of quality of vision. "Clarity of the lens capsule is a function of both the ability to clean out as much of the cortical material as possible, leaving the least amount of cellular debris to create scarring, and irregularity of the capsule," Dr. Kershner explains. "It is impossible to completely do that today. So those cells are going to continue to grow and proliferate and cause problems."
The designs of the lens optic edge and lens haptic junction are critical to delaying or preventing intracapsular opacification. "Studies have shown that the abrupt transition between the optic edge and the smooth transition between the haptic and the optic junction will impact whether or not the capsule epithelial cells will migrate into the central visual axis and interfere with vision. So lens design is important from that point of view," says Dr. Kershner.
The lens design is also critical to prevent diffractive interference. "When light is focused through the IOL optic, the stray light catches the edges of the optic and can be diffracted off of it. This causes diffractive interference," Dr. Kershner adds.
 |
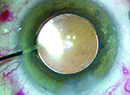
| AMO"s Tecnis has a modified prolate optic to reduce spherical aberration and improve visual quality. |
Best Performance
IOL lens material has long been a subject of research and debate. Dr. Kershner points out that with lens design the type of material can sometimes be less important than how the material is used. Thus, how the lens is designed and how it performs are two key indicators in evaluating a lens.
"Determining which is the 'best' lens is a function of good evidence-based clinical study and not what the manufacturers are telling us," says Dr. Kershner, who has no financial or proprietary interest in any lens.
He notes that bench studies, clinical tests and published peer-reviewed data can best teach about the differences in IOL design and performance. "With all things being equal, the one lens I would choose over any other right now would be the Tecnis, which has a modified prolate optic to intentionally reduce spherical aberration and improve visual quality, because it has been demonstrated in more than 30 clinical studies to improve contrast perception and image quality," Dr. Kershner says.
"Compared to the other most popular lenses out there—the AcrySof, the AcrySof higher-order aberration, the B&L aspheric and the like, it beats them all in terms of visual quality, optical quality, functional visual improvement, and clinical outcomes," he adds.
In one prospective study, conducted by Dr. Kershner, 221 eyes of 156 patients were randomly assigned to receive one of three IOLs: an aspheric IOL (Tecnis Z-9000, Advanced Medical Optics), a conventional silicone IOL (AA4207VF, Staar Surgical) or an acrylic IOL (AcrySof SA60AT, Alcon Surgical).1 He found that all three IOLs led to improved visual acuity after cataract surgery. However, the aspheric IOL provided a significant improvement in retinal image contrast and visual performance, measured by visual acuity and functional acuity contrast testing. This improvement was greatest in night vision and night vision with glare compared to the performance of conventional spherical silicone and acrylic IOLs.
Dr. Kershner cautions that heavy promotion doesn't correlate with high quality. Instead, solid scientific lens design makes the difference. "For example, AMO's OptiEdge design (the Sensar IOL with OptiEdge), which is designed specifically to reduce spectacle reflection, is a sophisticated, computer-driven design that has real science behind it," he says. "It has a round anterior and square posterior edge that are designed specifically to reduce internal reflection and glare, which is a serious problem with the acrylic lenses."
Numerous factors have been studied with regard to lens design and performance, including posterior capsule opacification, epithelial cell response, position change, higher- order aberrations and others.
Posterior Capsule Opacification
David Apple, MD, and colleagues have been researching lens design and PCO for many years. They reported on the Nd:YAG laser posterior capsulotomy rate of eight rigid and foldable IOL designs in a series of 5,416 pseudophakic human eyes obtained postmortem.2 They noted that newer IOL designs, such as the four modern, foldable IOLs manufactured from silicone and acrylic materials had significantly lower Nd:YAG laser posterior capsulotomy rates. Comparing foldable to rigid designs, the foldable IOLs were associated with a much lower Nd:YAG laser posterior capsulotomy rate.
The overall incidence of PCO and the incidence of Nd:YAG laser posterior capsulotomy is rapidly decreasing from rates as high as 50 percent in the 1980s to early 1990s, the group concluded.
In another comprehensive study,3 Dr. Apple and colleagues evaluated the influence of IOL material and design on the outcome of postoperative lens epithelial cell proliferation within the capsular bag after cataract surgery in 5,079 human globes containing rigid and foldable posterior chamber IOL styles commonly implanted in the United States.
They concluded that control of postoperative intracapsular cellular proliferation is important in avoiding PCO, anterior capsule opacification, and interlenticular opacification, or opacification within the capsular bag related to piggyback IOL implantation. IOL material and design are important factors influencing the outcome of these complications.
Recently, a study at Hayashi Eye Hospital, Fukuoka University, in Japan compared the degree of PCO and visual function in 75 patients implanted with an acrylic IOL with a sharp posterior optic edge, with that of eyes implanted with an IOL with a rounded-optic edge.4
The researchers found that the degree of PCO in eyes with an acrylic IOL with a sharp posterior optic edge is significantly less than that in eyes with a rounded-edge IOL, and the sharp-edge optic led to better contrast sensitivity with and without glare.
In the randomized clinical trial, one group of subjects received an acrylic IOL with a sharp edge (Sensar AR40e, AMO) in the left eye and an acrylic IOL of the same optic material and loops but with a rounded-edge optic (Sensar AR40) in the right eye. The other group received the sharp-edged IOL in the right eye and the rounded-edge IOL in the left eye. Sixty-nine patients (92 percent) completed follow-up.
The mean PCO value in the sharp-edge IOL group was significantly less than that in the rounded-edge IOL group throughout the follow-up period. The incidence of Nd:YAG capsulotomy also was significantly lower in the sharp-edge group than in the rounded-edge group. No significant difference was found in mean visual acuity during the 24 months of follow-up. However, contrast visual acuity with and without glare was significantly better in the sharp-edge group than in the rounded-edge group at 24 months after surgery.
 |
 |
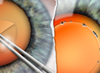
| The Sensar IOL with OptiEdge is designed specifically to reduce spectacle reflection. |
Another group at the Medical University of Vienna, in Austria, compared the intensity of capsule opacification between the sharp- and the round-optic edge variants of an open-loop hydrophobic silicone IOL in a randomized, controlled, double-blind clinical trial.5 Fifty-one patients (102 eyes) had cataract surgery in both eyes and received a Microsil IOL with a sharp-optic edge design (model S) in one eye and a Microsil IOL with a round optic edge design (model R) in the fellow eye. Both IOLs had an identical haptic design (nonangulated PMMA) and silicone optic material.
Five years postoperatively, the sharp-edged silicone IOL showed less regeneratory posterior capsule opacification (rPCO) and fibrotic PCO (fPCO) than the round-edged IOL. There was no significant difference in anterior capsule opacification between the two IOL styles.
A separate study at the same university compared the PCO-inhibiting effect of a three-piece PMMA IOL with a sharp-optic edge design with that of the round-edged version of the same IOL in 32 patients (64 eyes) during a five-year period.6 Each patient had phaco in both eyes and received a sharp-optic edge PMMA IOL in one eye and a round-optic edge PMMA IOL in the fellow eye.
The investigators found that, compared with the round-edge version, the sharp-optic edge design of the three-piece PMMA IOL led to significantly less PCO at one year, three years and five years after surgery. However, the sharp-optic edge did not lead to complete PCO prevention during the follow-up period.
LEC Response
A study in Ontario, Canada, evaluated and compared the lens epithelial cell (LEC) response and capsule dynamics in vivo after implantation of a square-edged and a round-edged silicone IOL in a fellow-eye study using high-magnification digital photography.7
After phaco, a square-edged silicone IOL (SoFlex SE, Bausch & Lomb) was implanted in 25 patients who had previously had a conventional round-edged version of the same IOL (SoFlex Li61U, B&L) implanted in their contralateral eye within a six-week period.
At one week, both designs had evidence of LEC migration along the posterior capsule. At one month, both IOL designs showed 360 degrees of anterior and posterior capsule adhesion to the edge of the optic. At one month, however, migrating LECs encountered a "damming" effect at the square posterior edge but not at the round edge. A thin fibrotic ring began to form around the SoFlex SE edge at two months and was complete for 360 degrees at three months.
At nine months, there was no evidence of LEC migration beyond this ring. With round-edged IOLs, however, the fibrotic ring never fully formed and LEC migration continued posterior to the optic. There was a tight capsule shrink-wrap effect with the square-edged IOLs with the fibrotic ring, allowing minimal IOL movement between one month and nine months. The round-edged IOLs tended to decenter and rotate. Anterior capsulorhexis contraction was greater at every time point with the round-edged IOLs than with the square-edged IOLs.
Position Change
Still another group at the Uni–versity of Vienna conducted two trials assessing the effect of optic edge design and optic-haptic angulation of open-loop IOLs on postoperative axial movement and the final position of the optic by measuring the anterior chamber depth during the first postoperative year.8
In the first study, a three-piece silicone IOL with nonangulated modified C-loop haptics (MicroSil) was implanted in 78 eyes of 39 patients; patients were randomized to receive a round-edged optic IOL in one eye and a sharp-edged optic IOL in the other eye. In the second study, a foldable, three-piece acrylic IOL with modified 10-degree angulated J-loop haptics (AcrySof MA60BM, Alcon) was implanted in 32 eyes of 32 patients.
 |
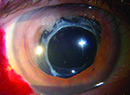
| The AcrySof SA60AT acrylic IOL. |
In eyes with a nonangulated silicone IOL, there was a significant postoperative change in ACD with both sharp-edged and round-edged designs. There was forward movement of both IOL designs in the first week, with no significant difference between the two models. From one week to three months, there was backward movement of IOLs of both designs, with the sharp-edged IOL moving a significantly greater amount. From three months to one year, IOLs with both optic edge designs moved slightly backward.
Sixty-six percent of angulated IOLs showed continuous but variable forward movement and 34 percent, backward movement.
The group concluded that the optic edge design influenced postoperative axial optic movement and, thus, had an impact on the development of postoperative refraction (refractive shift, deviation from target refraction). The influence of optic-haptic angulation proved to be significantly greater and more variable than edge design.
Higher-Order Aberrations
Research at Nara Medical University, Nara, Japan looked at the influence of IOL optical design on higher-order aberrations in a study of 60 eyes that compared two IOL optical designs.9 Types of IOL optical designs included: more posteriorly curved biconvex shape (AcrySof MA30BA, Alcon) or more anteriorly curved biconvex shape (AcrySof MA30AC). All patients had best corrected visual acuity better than 20/25. Higher-order aberrations were measured using a Hartmann-Shack aberrometer at 4.0-mm and 6.0-mm wavefront aperture diameters.
At 4.0-mm aperture diameters, there were no differences between the two groups in HOAs in the cornea and the whole eye. At 6.0-mm aperture diameters, MA30AC eyes had a smaller amount of spherical-like aberrations than MA30BA eyes; however, there were no significant differences between the two groups in coma-like aberrations and total aberrations in the whole eye. At 6.0-mm aperture diameters, there were no differences between the two groups' HOAs in the cornea. The optical design of the spherical IOL influenced the spherical-like aberrations in the whole eye, and this may reduce retinal image quality, they concluded.
Real Life Differences
The majority of patients today who undergo cataract surgery—regardless of the IOL design—are pleased with the results. However, even better outcomes are possible if surgeons ask their patients the right questions and perform contrast and glare testing, according to Dr. Kershner. While patients may not be familiar with technical terminology such as "glare," objective tests for glare can shed light on the effectiveness of the lens.
"First, I pushed for doctors to start testing for contrast because it can be done pretty easily and when you start testing, you find out what you really are doing," he says. "Then everyone is sold on using the Tecnis, because they find their contrast for their patients drastically improves."
He also would like to see more doctors testing for glare. "What we really need to do is test for both contrast and glare," he says. "We can do that very simply. There are a number of new computer-based glare testers coming onto the market and they work very quickly and really give surgeons a true, objective measure as to whether the patient has glare or not. You've got to test for glare, and if you do, you find that certain lenses do perform better than others."
1. Kershner RM. Image contrast and functional visual performance with aspheric, silicone, and acrylic intraocular lenses. Prospective evaluation. J Cataract Refract Surg 2003;29:1684-94.
2. Apple DJ, et al. Eradication of posterior capsule opacification: Documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology 2001;108:505-18.
3. Apple DJ et al. Influence of intraocular lens material and design on postoperative intracapsular cellular reactivity. Trans Am Ophthalmol Soc 2000;98:257-83.
4. Hayashi K, Hayashi H. Posterior capsule opacification in the presence of an intraocular lens with a sharp versus rounded optic edge. Ophthalmology 2005;112:1550-6.
5. Sacu S, et al. Long-term efficacy of adding a sharp posterior optic edge to a three-piece silicone intraocular lens on capsule opacification: Five-year results of a randomized study. Am J Ophthalmol 2005;139:696-703.
6. Findl O, et al. Long-term Effect of Sharp Optic Edges of a Polymethyl Methacrylate Intraocular Lens on Posterior Capsule Opacification A Randomized Trial. Ophthalmology 2005;112:2004-8.
7. Nixon DR. "In vivo digital imaging of the square-edged barrier effect of a silicone IOL." J Cataract Refract Surg 2004;30:2574-84.
8. Petternel V. "Effect of optic edge design and haptic angulation on postoperative intraocular lens position change." J Cataract Refract Surg 2004;30:52-57.
9. Taketani F, et al. "Influence of intraocular lens optical design on high-order aberrations." J Cataract Refract Surg 2005;31:969-72.