An Evolving Technology
Vance Thompson, MD, who practices at Vance Thompson Vision/Sanford Health in Sioux Falls, S.D., and is assistant professor of ophthalmology at the University of South Dakota School of Medicine, has been using WaveTec’s intraoperative aberrometer since its early days when he worked with the company to refine the algorithms and hardware. “For the first few years we just did data acquisition, comparing intraoperative power recommendations to postoperative outcomes for validation of accuracy and algorithm development,” he explains.
“The turning point for me came about two years into this process,” he says. “I had a patient who had undergone 3-D myopic LASIK at my practice, so I had all of the historical data. I plugged that data and the current measurements into the formulas and chose an appropriate lens power. During the surgery, however, the ORA device told me that if I put in the implant I had chosen the patient would end up with 3 D of myopia. We were going for a plano outcome, so I had a decision to make. I hadn’t been listening to the technology for two years; I was just acquiring data. Given that I had accurate historical data, I decided to put in the implant that I had chosen based on my calculations. The patient ended up with 3 D of myopia.
“Because her cornea was not thick enough for a conservative enhancement, she and I agreed that a lens exchange made the most sense,” he continues. “During the second surgery I took another ORA measurement; it told me to use the same power lens it recommended the first time. I did, and she ended up plano. So that was the beginning of my actually listening to the technology.”
Samuel Masket, MD, in private practice at Advanced Vision Care in Los Angeles and clinical professor of ophthalmology at the Jules Stein Eye Institute, Geffen School of Medicine, UCLA has been using intraoperative aberrometry for about four years. “I started with [WaveTec Vision’s] ORange,” he says. “Improved software in the ORA made it faster and easier to obtain readings. Next, ORA with VerifEye+ was introduced; it provides streaming information. Recently, as an alpha test site, I evaluated another new version with a dynamic reticle. I’ve used four generations of the device, each with changes making the unit faster, easier and more accurate.”
Dr. Masket notes that adding the dynamic reticle to the ORA instrument makes a big difference. “Having streaming information with the addition of VerifEye+ has been a big help in terms of accuracy when aligning a toric lens,” he says. “However, the surgeon still has to look away from the microscope to see it. With the dynamic reticle in the ocular of the microscope, the surgeon can see that information without looking away. I think that’s a really wonderful improvement.”
When IA Is Most Useful
Although some surgeons use intraoperative aberrometry on every cataract patient, most agree that it makes the most significant difference in three situations: when evaluating post-laser refractive surgery patients; when implanting a premium lens; and when correcting astigmatism.
 |
| The latest version of the ORA with VerifEye+ will add a dynamic reticle to the view through the microscope. (Image courtesy Samuel Masket, MD.) |
Dr. Thompson says this technology is also a cornerstone in his premium implant program. “With premium implants we’re trying to hit a specific refractive target meant for that particular implant because the patient’s goal is to go without glasses,” he says. “I’m trying to minimize the likelihood that I’ll need to enhance the patient—or at least get the patient close enough that if a laser enhancement is needed, the patient won’t need temporary glasses during the three months I wait between cataract surgery and the enhancement. So intraoperative aberrometry has become a very important part of my premium implant program.
“The third situation in which this technology is most useful is when implanting toric lenses,” he says. “In toric cases we do all of our preoperative calculations and place the implant lined up with the steep axis of corneal astigmatism, per the surgical plan. But then we take an intraoperative measurement and rotate the lens to the axis suggested by the VerifEye+ device. This has greatly improved my astigmatism outcomes with these implants.”
“Intraoperative aberrometry is particularly helpful in people receiving toric IOLs, because most of us don’t go to surgery with knowledge of posterior corneal astigmatism,” Dr. Masket points out. “We go to surgery with knowledge of the eye’s anterior corneal astigmatism, but the aphakic refraction is altered by posterior corneal astigmatism. Unless we have a good device to measure that—and most of us don’t—we don’t know for sure what the posterior cornea is contributing to the eye’s optics. Doug Koch has shown that there is, on average, a half-diopter of against-the-rule shift caused by the back surface of the cornea. For that reason I have found this technology very helpful to not only determine the axis of astigmatism, but also the magnitude of astigmatism.”
Another surgeon who has used this technology on many patients is David F. Chang, MD, clinical professor of ophthalmology at the University of California, San Francisco, and in private practice in Los Altos, Calif. Dr. Chang has used the ORA system with VerifEye+ regularly for more than 18 months, and has also used Clarity Medical Systems’ HOLOS IntraOp prototype during its development.
Dr. Chang says that in addition to post-refractive surgery eyes, he uses the ORA when implanting refractive IOLs and for eyes receiving limbal relaxing incisions. “I don’t have a femtosecond laser; therefore I perform all LRIs manually,” he explains. “One advantage of this is having the ability to monitor and titrate the LRIs intraoperatively with the ORA system. This is particularly helpful with a diffractive multifocal IOL. There have been cases where I did not plan to do an LRI because preoperative measurements showed only 0.5 D of cylinder; however, the pseudophakic ORA reading indicated closer to 1 D of against-the-rule cylinder. As a result, I went ahead and added one or two LRIs. Using the ORA with a pseudophakic eye, you can decide in real time whether to add a second incision or lengthen existing incisions. You cannot do this with a femtosecond laser.”
Dr. Thompson points out that he doesn’t use intraoperative aberrometry with every patient. “For instance, if someone is having traditional cataract surgery and he’s happy wearing spectacles and hasn’t had previous corneal surgery, I’m very comfortable doing the surgery based on my traditional calculations,” he says. “The formulas are quite good, and they’ve gotten better over time. But for all those other cases, I do use this technology. That includes individuals who have an A-scan that’s challenging to interpret, or who couldn’t sit up for a good-quality reading, or who have a big difference in readings between eyes. Whenever I have any concerns, it’s a nice tool to have at my disposal for reassurance. And it has made a difference; it has reduced our enhancement rate by about 20 percent in those patients who have the goal of being able to do a lot without glasses. It’s had a significant impact.”
What the Data Shows
When he first tried using the technology, Dr. Masket conducted a study to test its value. “I did 200 consecutive cases,” he says. “I wanted to see how intraoperative aberrometry would compare to the methods I had used in the past. At that point, we were measuring the eye with two instruments, the IOLMaster and the LenStar; then we’d run the data through five different formulas and I would choose an IOL power based on the results. For this study, my protocol was that if ORA said to change my previously decided IOP power, I would.
“To evaluate the effectiveness of the new technology, I looked for cases among the first 200 consecutive eyes that had all been implanted with the same lens design—a single-piece acrylic lens—and in which the patients had no prior corneal surgery, corneal pathology or other comorbidity that would reduce vision to less than 20/25,” he continues. “Out of the 200 cases, 131 eyes fit those parameters. Among those cases, I had changed IOL power in response to the ORA in 42.7 percent of the eyes. Then I compared the two groups: those eyes in which we changed the IOL power at the suggestion of the ORA device, and the 57 percent of patients where ORA and my methods agreed. I only looked at the resulting spherical error, not spherical equivalent, because toricity was not always addressed.
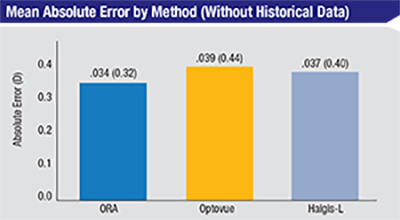 |
| In this group of 39 eyes with previous laser vision correction but no historical data, using the ORA produced better results, on average, than basing treatment on the Optovue or the Haigis-L formula. The difference between the groups, however, was not statistically significant. (Based on Fram, Masket, et al, 2015.1) |
Dr. Masket and co-authors Nicole Fram and Li Wang recently conducted a study comparing intraoperative aberrometry to his traditional methods for determining post-laser-vision-correction IOL power. “Traditionally in our office, when we know what excimer laser ablation was done, we’ve used the Masket formula to calculate the IOL power,” Dr. Masket explains. “That has worked well over the years. If we don’t have the information about the laser ablation, we have relied primarily on the Haigis-L formula.
“We wanted to see how new technology compared to this approach,” he continues. “The new technology we used in the study included ORA intraoperative aberrometry and RT-Vue OCT with total corneal power, or TCP. Our study, which was prepublished in Ophthalmology, included 39 eyes.1
“In terms of accuracy, the data found no statistically significant difference among any of the methods,” he says. “However, when we knew the previous laser treatment, the trend was toward better results with the Masket formula. If we didn’t have the laser treatment information, the trend with the remaining tools was toward intraoperative aberrometry being a little stronger, although statistically similar. As an example, we found that when using intraoperative aberrometry, 50 percent of patients were within ±0.25 D of the intended target and close to 75 percent were within 0.5 D. We think these outcomes are pretty remarkable, given that these were post-refractive-surgery eyes. Even though there was no statistically significant difference in the results, ORA stood up very well against the other methods.”
Dr. Chang co-authored a recent paper that compared ORA’s wavefront aberrometry refractive methodology to other formulae for IOL power accuracy in post-myopic LASIK and PRK eyes.2 “Using the ORA algorithm and intraoperative aphakic measurements, 67 percent of eyes were within 0.5 D of the target refraction, compared to 48 percent with Haigis-L, 50 percent with Shammas and 46 percent with the surgeon’s preoperative selection based on the ASCRS online calculator,” he says. “I tell patients that this doesn’t negate the challenges posed by having had prior LASIK, but it improves our batting average compared to other methods.”
Other Issues
Surgeons considering adopting this technology may have other concerns besides its effectiveness, including the possibility of lengthening the time required for cataract surgery and the possibility of false readings.
“Using this technology doesn’t take much time,” says Dr. Thompson. “When we first started using it it was slower, but it’s become very quick and intuitive. That’s mainly because of improvements in the acquisition speed, which is very fast in the latest generation. My part as the surgeon has never taken very long.”
“There is some learning curve for the surgeon and the OR staff, which adds more operative time initially,” agrees Dr. Chang. “The ORA VerifEye+ system is much faster at data acquisition than earlier generations. However, I do spend some time reviewing the ORA recommendation alongside my preop diagnostics before deciding on the final IOL power.”
“When you’re starting out, you might want to schedule a couple fewer cases for your first day of using it,” says Dr. Thompson. “But within a week or two you’ll be back to your regular volume and comfortable with the technology. You do have to get used to the fact that your working distance is slightly reduced, because the instrument fits under the microscope; if you aren’t careful, you can touch it with an instrument or your hand, and then you have to hand off the instrument and change gloves. But it doesn’t take long at all to get used to the smaller working distance.”
“There are many potential artifacts that can undermine the quality of the data,” notes Dr. Chang. “This is universally acknowledged. Corneal surface drying, ocular hypotony or over-inflation, poor fixation, and pressure from the lid speculum and drapes can cause misleading results. However, having a continuous display of refractive data allows you to assess the effect of patient fixation, intraocular pressure and lid speculum pressure in real time. This teaches the surgeon when erroneous artifacts are introduced, and how to better avoid them.”
Making the Most of IA
Surgeons offer these strategies to help make sure that intraoperative aberrometry provides you with the best possible results:
• Don’t expect to get out of doing preoperative calculations. “It’s not a good idea to go into the OR without a solid idea of the correct power and cylinder for the lens,” says Dr. Thompson. “Your calculations are critical. An electrical or software glitch is rare, but they can happen, and if they do you’re going to find yourself in a very uncomfortable situation.
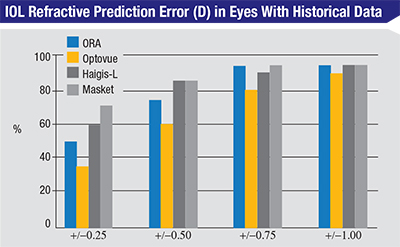 |
| In this group of 20 eyes with previous laser vision correction and historical data available, there was a trend toward more accurate results using the Masket method, although no method was statistically significantly superior. (Based on Fram, Masket, et al, 2015.1) |
Dr. Masket agrees. “I know there are surgeons who don’t use the formulas to calculate a likely IOL power, relying only on intraoperative aberrometry,” he says. “I would go to surgery with a plan and then use intraoperative aberrometry to refine it.”
• Remember that this technology is less helpful in patients who have had radial keratotomy. “Sometimes it’s hard to achieve good quality readings in these eyes,” notes Dr. Masket. “Among other things, we induce intraoperative change in the corneal shape in these eyes.”
• Make sure the patient is properly oriented with the instrument. “The patient should be oriented with the device in a vertical fashion in order for it to work its best,” says Dr. Thompson. “You need to decide whether you’re doing a superior or temporal incision. If you’re doing a superior incision, you want the instrument lined up with 90 degrees and 270 degrees, or 6 o’clock and 12 o’clock. If you’re doing temporal surgery, it should be lined up with 0 degrees and 180 degrees, or 3 o’clock and 9 o’clock. If you aren’t lined up perfectly, the instrument doesn’t know that, and it will affect the reading. Fortunately, the dynamic reticle helps us avoid that.”
• Monitor the intraocular pressure. “You don’t want too low or too high of a pressure during the reading,” says Dr. Thompson. “For that reason we use a Barraquer tonometer in surgery to make sure the pressure is around 20 mmHg. This becomes even more important in radial keratotomy cases because the corneal curvature can change rather dramatically with lower or higher pressure.”
• Be aware of intraoperative factors that can produce inaccurate readings. Dr. Masket notes that a number of things can cause inaccurate readings. “One may run into trouble with an irregular incision; if you overhydrate the incision; if the corneal surface is irregular; if there’s been too much drying because of insufficient hydration; if the patient isn’t cooperative and is not looking at the target; or if the speculum or other instruments are pushing on the eye,” he says.
Dr. Thompson concurs. “Overhydrating the incision will induce changes in the astigmatism reading,” he notes. “For that reason, thinking about your incision architecture ahead of time is very important. You want to have a self-sealing incision that requires minimal or no hydration. Corneal power readings can also be altered by a fluid meniscus. So, right before taking the reading I moisten the cornea and dry the fornices with a Weck-Cel sponge to make sure there’s not an excess amount of fluid. Once you have dry fornices and a pristine, moist corneal surface, you take your measurement.”
• Use an ophthalmic viscosurgical device with a low index of refraction. “We recently completed a study to determine the effect of leaving the OVD in the eye when readings are obtained,” says Dr. Masket. “What we found was that when using an OVD that has a low index of refraction close to that of BSS, the refractive readings will be the same. But with an OVD with a higher index of refraction, the intraoperative aberrometry readings will be in error. That may lead one to undercorrect the optical error.”
• Adjust your use of viscoelastic during the reading based on the purpose of the reading. “I typically remove the viscoelastic for my toric measurements,” explains Dr. Thompson. “You want to make the measurement with a consistent medium—just BSS, not a mix of some BSS and viscoelastic. On the other hand, in the aphakic state I leave the eye filled with viscoelastic when we take the measurement because it’s usually a post-refractive or premium cataract case. Also, when you’re measuring with viscoelastic in the eye, make sure there are no bubbles in the pupil area. Those can distort the reading.”
• Don’t blindly follow an ORA recommendation if it contradicts your preoperative decision. Dr. Chang notes that even with this technology there is still a lot of “art” to determining the optimal spherical and toric power and axis. “Many of us employ multiple IOL formulae and different preoperative diagnostic measures of cylinder,” he says. “One should never blindly follow the ORA recommendation if it contradicts the preoperative diagnostics. Instead, it should be used as an adjunct or tie-breaker. Now that we’ve been using the ORA, I am impressed with how often I’m undecided between a choice of two spherical or toric powers, or two different astigmatic axes. In these situations I have found ORA to be correct about four out of five times when used as a tie-breaker.”
What the Future May Hold
As with any technology, there’s room for improvement. For example, Dr. Thompson notes that the technology reaches its limits with severely aberrated corneas. “The more aberrated a cornea is, the less you can rely on the measurements,” he says. “If you encounter a highly aberrated eye, you have to use your own clinical judgment. Of course, if the eye is extremely aberrated, the instrument flashes a red light and won’t give a reading. I look forward to reaching the point at which there are no red lights and you get an accurate measurement even in highly aberrated corneas.”
Other systems, such as Clarity Medical Systems’ HOLOS IntraOp wavefront aberrometer, are in the pipeline. “The HOLOS system will also display astigmatic data in real time in order to titrate LRIs or toric IOL positioning,” notes Dr. Chang, who used the HOLOS prototype during its development. “However, it will not be able to calculate the recommended spherical IOL power, at least initially.”
Dr. Thompson foresees a day when this technology will be used in concert with Calhoun Vision’s light-adjustable lens, which he has also worked with during its development. “That technology will allow us to adjust the power of the implant after it’s inside the eye,” he notes. “Let’s say a patient has a final refractive error of +0.25, -0.3, axis 180, and sees 20/20 uncorrected—but with this best-corrected refraction the patient sees better. We typically would not treat that amount of refractive error on the cornea because the limits of biological healing. But with the light-adjustable lens you’re changing a polymer inside the eye, so we make corrections this small all the time. You’d be amazed how crisp someone’s vision becomes when you take her to a 0 correction without touching her cornea.
“Ultimately, I see these technologies working together,” he says. “I can imagine a day when we’ll use intraoperative aberrometry to determine our very best refractive endpoint. Then, whatever final changes occur because of biologic variables such as incisional healing, corneal healing, capsular bag contraction and so on, we’ll do the final adjustment with the Calhoun lens.”
Is It Worth the Investment?
No matter how good a given technology may be, surgeons still have to decide whether the technology is worth the cost of purchasing and implementing it.
“In my opinion, this technology is definitely worth the cost,” says Dr. Thompson. “In fact, I would pay more for it because of all the value it brings me. We used to have this technology in one room of our two-room surgical suite. Now we have it on both microscopes because we use it so frequently. I don’t ever want to operate in an OR that doesn’t have one.”
Dr. Thompson sees intraoperative aberrometry as one of the three most impactful technologies in current cataract surgery. “In terms of the most significant advancements in our technology,” he says, “I’d list the amazing advanced implants we now have available to us, the excimer laser that allows us to enhance the final refractive state if necessary, and third, intraoperative aberrometry. I like to use the femtosecond cataract laser, and I prefer it to manual cataract surgery. However, I can do quite accurate cataract surgery without it. I can’t document refractive power intraoperatively without an aberrometer. It’s an important tool for taking refractive cataract surgery to the next level. If I had to choose between the femtosecond laser and the aberrometer, the aberrometer would be my choice. Of course, I’m glad I don’t have to make that choice.”
Dr. Chang agrees. “For improving our refractive results, I think intraoperative aberrometry adds significant value—certainly more than a femtosecond laser would,” he says. “Because it offers an IOL recommendation based on intraoperative aphakic and pseudophakic refractions, it provides extra data that we previously didn’t have. At a minimum, it provides confirmation of the preoperative plan. When used as a tie-breaker in the proper context, it usually steers us toward the better result. Finally, for our demanding post-LASIK patients, it appears to be the single best methodology that we currently have—particularly when the historical data is unavailable, as is so often the case. However, you should not expect it to replace or circumvent your experience and judgment, or extensive preoperative testing.”
Dr. Masket admits that using intraoperative aberrometry does add time to the surgery, and the surgeon must follow specific protocols to get accurate results. However, he feels it’s worth the time and effort. “Using this technology makes me more confident of my IOL power selection,” he says. “When I sit and look at the patient’s chart prior to surgery to decide what power to use, if I’m on the fence about which way to go, I don’t worry. I know the ORA will help me make that decision. I have no doubt that surgeons who are compulsive about accuracy, who are reproducible in their incision construction and are discerning about correcting astigmatism, would benefit from this technology.” REVIEW
Dr. Thompson is a researcher and consultant for Calhoun, WaveTec and Alcon. Dr. Chang is a consultant for Clarity Medical Systems; he has no financial interest in WaveTec or Alcon. Dr. Masket has been a consultant to WaveTec, which also provided support for some of his research. He is currently a consultant to Alcon.
1. Fram NR, Masket S, Wang L. Comparison of Intraoperative Aberrometry, OCT-Based IOL Formula, Haigis-L, and Masket Formulae for IOL Power Calculation after Laser Vision Correction. Ophthalmology 2015;122:6:1096-101. doi: 10.1016/j.ophtha.2015.01.027. Epub 2015 Mar 10.
2. Ianchulev T, Hoffer KJ, Yoo SH, Chang DF, Breen M, Padrick T, Tran DB. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology 2014;121:56-60.
The published version of this article suggested that the HOLOS system was not approved in the United States. Clarity Medical Systems points that the HOLOS is a Class I medical device and thus not subject to the regulatory process.



