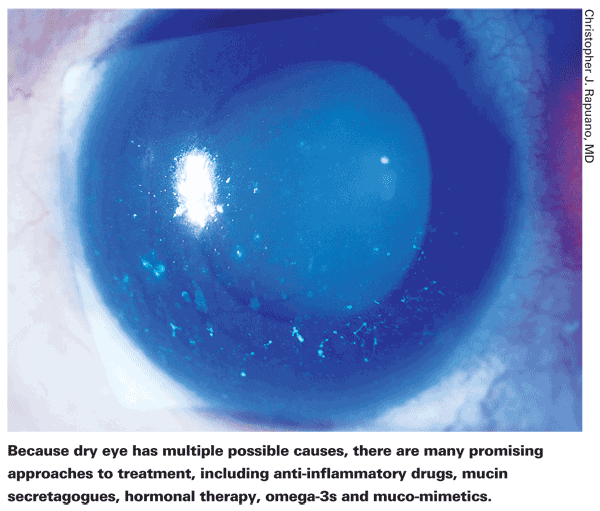
Treating dry eye, once a topic of little interest, has now become the focus of a tremendous amount of research. To date, only one new product going beyond the category of artificial tears has been approved in the
Here, in addition to a list of some of the most promising drug candidates in the pipeline, two people with extensive experience in this area discuss the state of dry-eye drug development and where the field may be headed.
Six Approaches to Treatment
Many of the clinical trials for potential dry eye treatments are being conducted under the auspices of Ora, Inc., in Andover, Mass. George Ousler, director of dry-eye research at Ora, notes that the firm developed the CAE, or controlled adverse environment, more than a decade ago to allow a more standardized and well-controlled approach to studying dry eye. "The CAE allows us to regulate humidity, temperature, air flow and visual tasking," he explains. "In addition, our systematic approach involves a myriad of novel diagnostic technologies including the ocular protection index, interval visual acuity decay, tear-film break-up time and blink pattern. We minimize the background noise that's associated with the typical dry eye environmental study."
Mr. Ousler says there are currently six categories of drugs for treating dry eye under investigation. "The category that seems to be generating the most interest right now is anti-inflammatories," he says. "There's a lot of debate about which comes first: Does inflammation produce dry eye, or does dry eye produce inflammation? Either way, if you use an anti-inflammatory you'll reduce the inflammation. You may not be treating the underlying disease, but you're stopping the irritation; that will eventually allow the ocular surface to become more healthy." He adds that many of the anti-inflammatories under investigation are based on existing systemic medications that are being reformulated for topical delivery to the eye.
Christopher J. Rapuano, MD, director of the cornea service and coodirector of the refractive surgery department at Wills Eye Institute and professor of ophthalmology at Jefferson Medical College of Thomas Jefferson University in
"The only prescription medication we have that seems to work well in a lot of patients—though not as many as we'd like—is Restasis," he continues. "But Restasis doesn't work for everybody, and it's irritating for some patients. I'd love to evaluate another form of cyclosporine, or some non-steroidal anti-inflammatory to see whether it would work in those patients who don't respond to Restasis or can't tolerate it." He notes that the existing non-steroidal anti-inflammatory drops are very problematic. "They can cause melting and scratches and poor healing," he says. "But maybe someone can create a formulation that will eliminate these concerns."
A second category is mucin secretagogues. "These are intended to increase secretion of mucin from goblet cells," explains Mr. Ousler. "In dry eye, we see the density of the goblet cell population in the eye shrinking with disease.
Additionally, the quality of the mucin that's being secreted can decrease. Trying to address this is complicated by the fact that we currently know of at least 20 different mucins. Nevertheless, companies are hoping that mucin secretagogues will increase goblet cell density, and/or improve the quality of the mucin being secreted."
Some of the drugs in this category are currently approved for treatment of gastric ulcers or have been used to treat cystic fibrosis. Diquafasol, which is a mucin secretagogue, is now approved in
A third category of potential dry-eye treatment is hormonal therapies. "As you know, dry eye is most common in postmenopausal women," says Mr. Ousler.
"Research has found that postmenopausal hormonal changes seem to decrease the quality of lipid secretions from the meibomian glands. So companies are looking at topical testosterone as a potential dry-eye treatment.
"There probably is some hormonal base to dry eye," observes Dr. Rapuano. "However, I don't think we have a good handle on the connection at this point. There's great promise there, but I'm not convinced that this approach is ready for prime time."
Other promising dry-eye drug categories include omega-3 fatty-acid-based compounds. "Omega-3s seem to have a positive effect on dry eye," Mr. Ousler notes. "At the moment, these are in the form of oral supplements, and many patients report that they help."
 Dr. Rapuano agrees. "There's good evidence that omega-3 fatty acids help in some patients with dry eye and some patients with blepharitis," he says. "Again, there are no major studies that show that they help in a huge proportion of patients. But we know that omega-3s help with heart disease and decrease inflammation, so I think there is a place for this class of drug. If someone could create a topical omega-3 formulation that works, that would be great."
Dr. Rapuano agrees. "There's good evidence that omega-3 fatty acids help in some patients with dry eye and some patients with blepharitis," he says. "Again, there are no major studies that show that they help in a huge proportion of patients. But we know that omega-3s help with heart disease and decrease inflammation, so I think there is a place for this class of drug. If someone could create a topical omega-3 formulation that works, that would be great."
A fifth category is muco-mimetics. "These are synthetically produced mucins," Mr. Ousler explains. "Hopefully, they can serve some of the functions of natural mucins and thus provide some dry-eye relief. And of course, a final category would be the ever-improving barrier-function agents, such as Alcon's Systane Ultra and Bausch + Lomb's Soothe XP."
Drugs in the Pipeline
Here's a brief sampling of some of the more promising drugs currently in the pipeline:
• Cyclokat (
• CF-101 (CAN-FITE
• RX-10045 (Resolvyx,
RX-10045 is a synthetic analog of RvE1, a naturally occurring resolvin. It successfully completed a randomized, placebo-controlled, 232-patient Phase I/II trial in August 2009. In that trial it produced dose-dependent, statistically significant improvement in the primary endpoints for both the signs and symptoms of dry eye, including dryness, stinging, burning, grittiness and ocular discomfort. The onset of symptom relief occurred within the first week of treatment, and symptoms continued to improve over the course of the 28-day study. The drug was generally shown to be safe and well tolerated.
 RX-10045 was scheduled to begin Phase III testing sometime this year.
RX-10045 was scheduled to begin Phase III testing sometime this year.
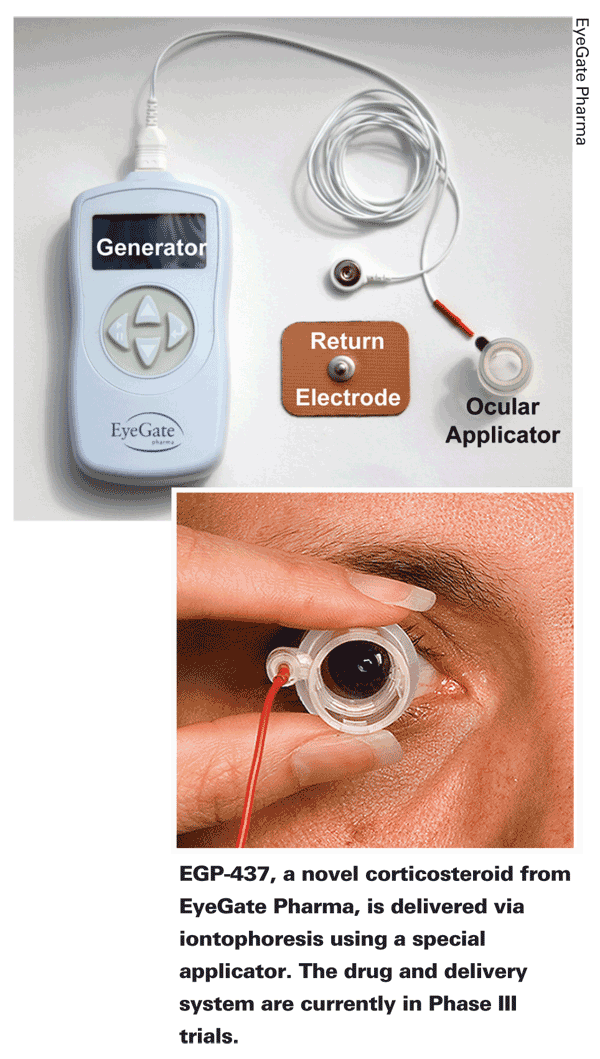
• EGP-437 (EyeGate Pharma,
The Phase III trial, which Ora is running, launched in June 2010.
• Remura (Ista Pharmaceuticals,
(Twice-daily Xibrom will be replaced by the newly approved once-a-day bromfenac product Bromday during the next few months.) Phase III trials of low-dose bromfenac as a treatment for dry eye are under way as of September 2010. Ista plans to conduct four randomized, double-masked, placebo-controlled studies under a special protocol assessment agreed upon with the Food and Drug Administration; the studies will involve more than 30 sites in the United States. Two concentrations of bromfenac will be tested (both lower than Xibrom's 0.09% concentration). To meet FDA guidance on drugs for chronic dosing, the company expects to conduct both a six-month and a 12-month safety study. According to the Ista website, the company hopes to report efficacy results by mid-2011.
• SAR 1118 (SARcode, San Francisco) SAR 1118 is an anti-inflammatory, LFA-1 antagonist that, according to the company, can be targeted against a broad range of ocular inflammatory conditions including dry eye, uveitis and diabetic macular edema. Its target, LFA-1, regulates T-cell adhesion, migration and proliferation, as well as cytokine production, all of which are associated with reduced tear-film quality and ocular surface inflammation. SAR 1118 has demonstrated significant potency in in vitro models in inhibiting cell adhesion, cytokine production and cellular proliferation.
Results from a Phase II study were reported in May 2010. The randomized, multicenter, double-masked study, involving 230 subjects, demonstrated clear improvements in both signs and symptoms of dry eye at 12 weeks. SAR 1118 was well-tolerated; no serious ocular adverse events were reported.
• AzaSite (Inspire Pharmaceuticals,
In March 2010, Inspire announced the results from two Phase II clinical trials comparing AzaSite to vehicle for the treatment of anterior blepharitis. In a four-week trial, AzaSite produced significant improvement in a number of blepharitis signs and symptoms at various time points, but not in the primary endpoint of mean lid margin hyperemia. A two-week trial found no statistically significant improvements in the AzaSite group. AzaSite was well-tolerated in both trials. The next step in the program will be refining the clinical trial design for subsequent trials, including some additional Phase II trial work expected to begin in late 2010.
The company will also be pursuing trials in posterior blepharitis, and anticipates pursuing eventual Phase III trials.
• Lancovutide (Lantibio,
• Tasocitinib (Pfizer) Tasocitinib is an immunosuppressant and anti-inflammatory, under investigation in multiple trials for multiple indications, including transplant rejection, inflammatory bowel disease and psoriasis. A Phase II study for treatment of dry eye is currently recruiting about 280 participants; the estimated study completion date is listed as March 2011.
• Rebamipide (Acucela, in partnership with Otsuka Pharmaceutical Co.) Rebamipide is an amino acid derivative of 2(1H)-quinolinone that causes mucus secretion; it's been used for mucosal protection, healing of gastroduodenal ulcers and treatment of gastritis. It works by enhancing mucosal defense, scavenging free radicals and temporarily activating genes encoding cyclooxygenase-2. Data from a recent multicenter, randomized, double-masked, parallel-group study of rebamipide involving 308 participants, conducted in
Ora is running two Phase II clinical trials for Acucela; one is a controlled adverse environmental study; another one is looking at central corneal staining. The estimated completion date is listed on the clinicaltrials.gov site as October 2010. (A previous Phase III trial by Novartis was not completed.)
• RGN-259 (RegeneRx Biopharmaceuticals,
• IL-1-Ra (Massachusetts Eye and Ear Infirmary,
• AL-43546 (Alcon) A sodium hyaluronate-based ophthalmic solution. Alcon's pipeline page and clinicaltrials.gov both note that a Phase II trial has been completed in
Why So Few Survive
Anyone observing the drug pipeline in recent years has undoubtedly noticed that many dry-eye drugs make it partway through clinical testing and then disappear.
"There have been a number of successful Phase II programs," observes Mr. Ousler. "But there are factors besides not achieving endpoints that can keep a new drug from moving forward. There may be a formulation problem, an intellectual property issue, a refocusing of funding within a company or safety issues. But with some of the drugs that haven't made it, the clinical evidence of effectiveness was definitely there."
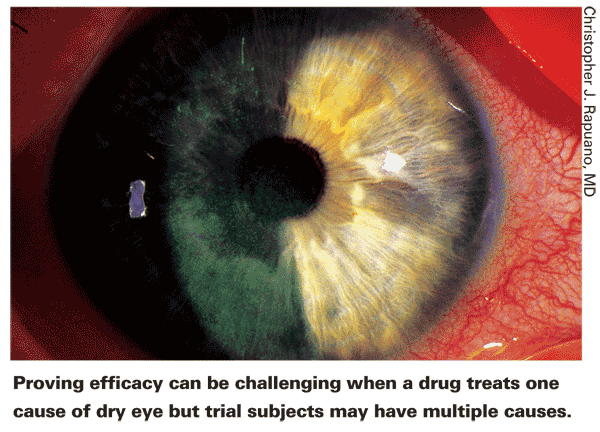
Dr. Rapuano notes another factor that derails some tests. "Dry eye is multifactorial," he points out. "I think that's one reason studies often don't show as strong an effect as you might expect. A given drug—for example, a mucin secretagogue—might be very effective in the 20 percent of people that it's the right medicine for. But the 80 percent for whom mucin is not the issue won't show any benefit. If we could identify the people with the exact problem the drug is treating, the clinical trial results might be spectacularly good."
With that in mind, Dr. Rapuano envisions a future in which we'll be able to determine the exact problem and address it. "Maybe 10 years from now we'll have five different products, and we'll have a little assay," he says. "We'll dip something into the patient's tears; we'll see that the patient is missing factors A, C and G, so we'll give the patient three drops. Or, we'll just order up tears containing A, C and G from the pharmacy—they'll put the appropriate three ingredients into the bottle.
 "It's the same problem with the current selection of artificial tears," he continues. "Some patients swear by one and hate the other; with other patients it's the other way around. I don't think they're crazy; I think there are components in one eye-drop that are helpful for some pa-tients and not helpful for others.
"It's the same problem with the current selection of artificial tears," he continues. "Some patients swear by one and hate the other; with other patients it's the other way around. I don't think they're crazy; I think there are components in one eye-drop that are helpful for some pa-tients and not helpful for others.
"For that reason, I give people several samples and say, 'Whichever one works the best for you, that's the one I want you to buy,' he adds. "Right now, we don't necessarily have the tools to tell which is going to be most helpful for which patient."
We've Come a Long Way
"It seems to me that in the past three or four years we've seen more potent agents being evaluated, with novel mechanisms of action," notes Mr. Ousler. "Some companies are looking at new chemical entities. Furthermore, we're seeing improved study designs and technologies. And I think our understanding of dry eye has improved quite a bit just in the past three years. In addition, we're seeing new delivery methods such as iontophoresis, subconjunctival injections and inserts, which may help with efficacy and duration of action. I think we may see some approvals in the next few years. Dry eye is definitely on the priority list for many researchers and companies."
Dr. Rapuano agrees. "There's a lot more interest in dry eye right now than there was in 2000," he says. "Ten years ago, half of these drug classes for treating dry eye were unheard of, or at least not being looked into. Part of the reason this has changed is that we have a better understanding of dry eye, and part is that medical science has improved. And, of course, the popularity of LASIK and premium intraocular lenses has placed added importance on dry eye; surgeons know that it can affect surgical outcomes.
"I also think that drug company marketing has been beneficial," he adds. "Of course, direct marketing has pros and cons, but in this case it's increased awareness of dry eye in the ophthalmic community. Doctors and patients are more attuned to dry eye, and that creates a snowball effect. If patients and doctors are more attuned, then companies are willing to devote more R&D to this group of patients. And when they're investing in it, they want patients and doctors to be aware of it.
"Today, a doctor ignores his pa-tients' dry-eye problems at his own peril," he concludes. "You can't do that too much and maintain a thriving practice."



