 |
|
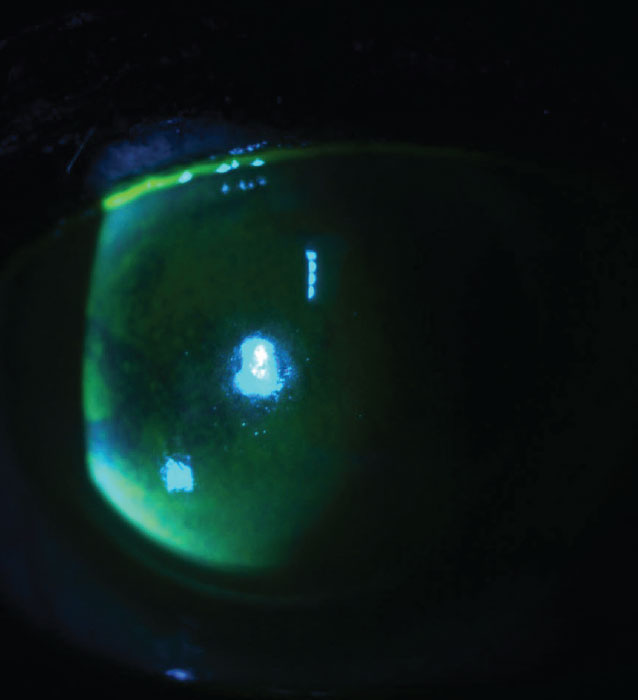
Figure 1. A patient with classic signs of benzalkonium chloride toxicity, with a confluent, almost limbus-to-limbus, pattern of punctate staining. |
Glaucoma drops, especially those containing preservatives, may exert a number of well-documented negative effects on the ocular surface. These topical agents can also affect visual quality by way of their effects on the diffraction, reflection, scatter and filtration of light. Here, I’ll discuss several different classes of glaucoma medications, how they affect the refractive status of the eye and how these effects translate into actual symptoms patients may complain about.
Benzalkonium Chloride
As the most ubiquitous preservative in topical glaucoma medications,1 benzalkonium chloride is something all glaucoma specialists and ophthalmologists deal with on a daily basis. BAK exerts some corneal and conjunctival epithelial toxicity in the form of cell loss, disruption of tight junctions and even apoptosis,1 but the exact mechanism of that toxicity is still unclear.
There’s evidence that BAK inhibits mitochondrial oxygen consumption in human corneal epithelial cells in a dose-dependent manner, with a steep decline in oxygen consumption with increasing concentrations of BAK.2 This effect is enhanced in cells bearing mutations in mitochondrial complex-1, which is implicated in some forms of primary open-angle glaucoma, as well as other eye diseases.2,3 So, there’s even a potential that BAK toxicity could be amplified in some glaucoma patients compared with the general population.
Classic signs of BAK toxicity include superficial punctate keratopathy (Figure 1), conjunctival hyperemia, staining and follicles, blepharitis, increased osmolarity and reduced tear production and tear breakup time.4
Placido-based topography does a good job of demonstrating the refractive effect of these punctate erosions. This typically manifests as disrupted mires, which is indicative of irregular astigmatism, an increase in backward light scatter and higher-order aberrations.5 All of these factors could in turn lead to inaccurate biometry. These patients may experience blurred vision, glare and reduced contrast sensitivity—and postop unhappiness if they’ve had cataract surgery based on these measurements.
ROCK Inhibitors
Though they’re used as therapeutic agents in corneal disease and for treating glaucoma, there’s still much we don’t understand about ROCK inhibitors and their effects on the cornea. Two notable side effects of the drug include honeycomb edema and refractive shifts.
Honeycomb edema is a classic finding with ROCK inhibitor use, usually occurring within a week or two of starting the drug. It tends to be seen in corneas with already compromised endothelium,6 such as from Fuchs’ dystrophy, prior keratoplasty, incisional glaucoma surgery or ACIOL implantation, but it may affect corneas with normal-appearing endothelium as well.
 |
|
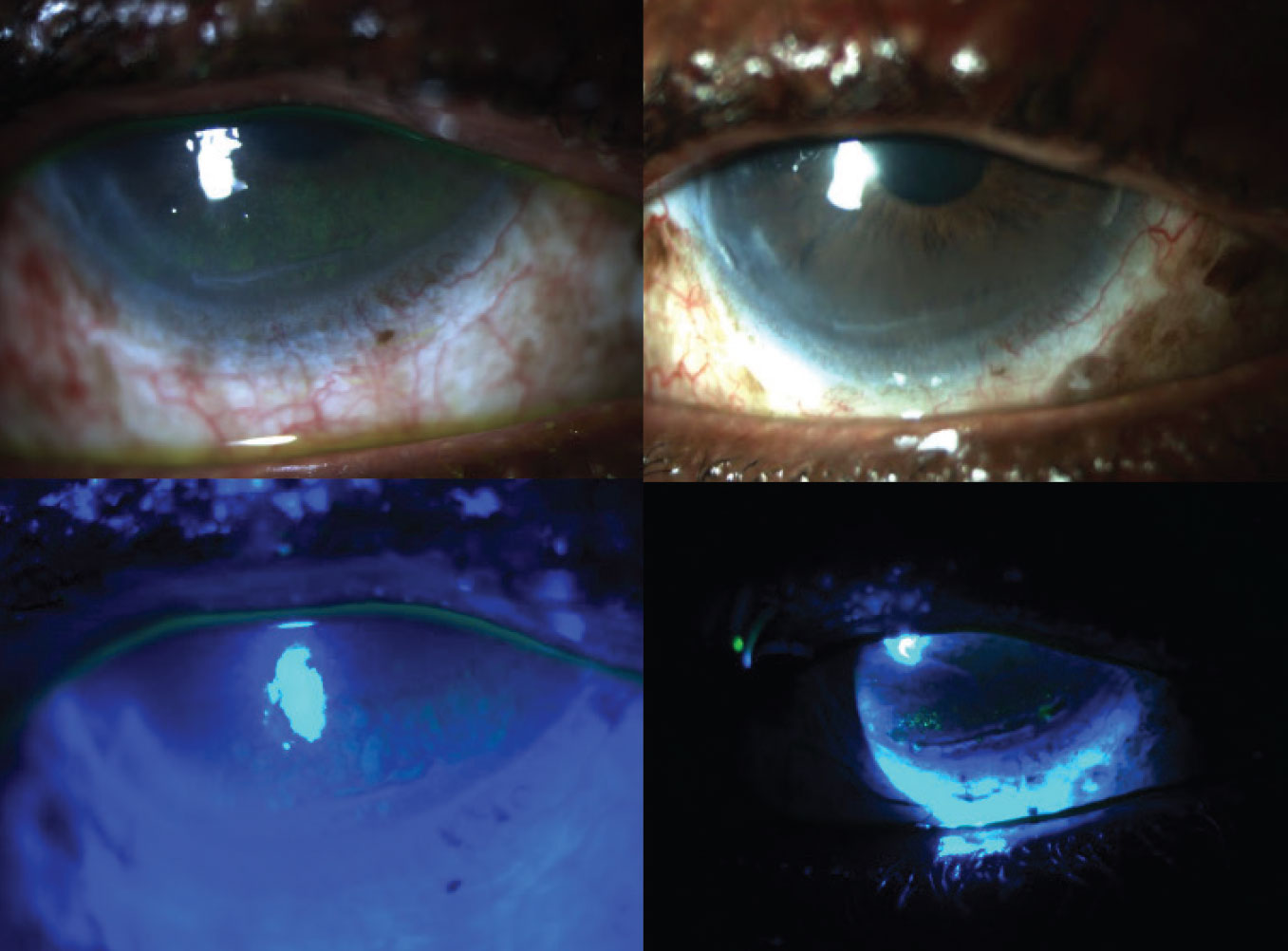
Figure 2. New-onset honeycomb edema (left) from ROCK inhibitor use that resolved (right) after withdrawing netarsudil in a severe glaucoma patient. |
Figure 2 shows new-onset honeycomb edema (left) in a patient with severe glaucoma who has had multiple prior procedures. The edema began after starting Rhopressa (netarsudil 0.02%) in 2019 when it was new to the market. At that point, honeycomb edema had only been described with the use of ripasudil 0.4% (Glanatec), which wasn’t and still isn’t available in the United States. The honeycomb edema resolved (right) with discontinuation of the drug.
There have also been reports of significant myopic and hyperopic refractive shifts even in the absence of edema that reverse once the drug is withdrawn. In one case, a 72-year-old man with primary open-angle glaucoma, prior cataract surgery and prior radial keratotomy with no history of refractive shift in the past 20 years reported reduced visual acuity one month after starting fixed-dose combination netarsudil/latanoprost.7 He had an approximately 1.5-D shift in both eyes, associated corneal contour changes and no epithelial bullae or edema. His refractive error and corneal contour returned to baseline after he stopped netarsudil/latanoprost and was switched to timolol.
In another case, after adding Rhopressa to an existing regimen of timolol and latanoprost, a 4-year-old girl with secondary open-angle glaucoma developed 6.5 D of corneal flattening, which reversed after stopping netarsudil.8 In the case report, the researchers noted the role of rho kinase inhibitors in corneal endothelial healing but added that if an excess of cell proliferation were to occur, that might induce stromal cells to abnormally secrete enzymes or proteins that could lead to corneal fibrosis and subsequent flattening.
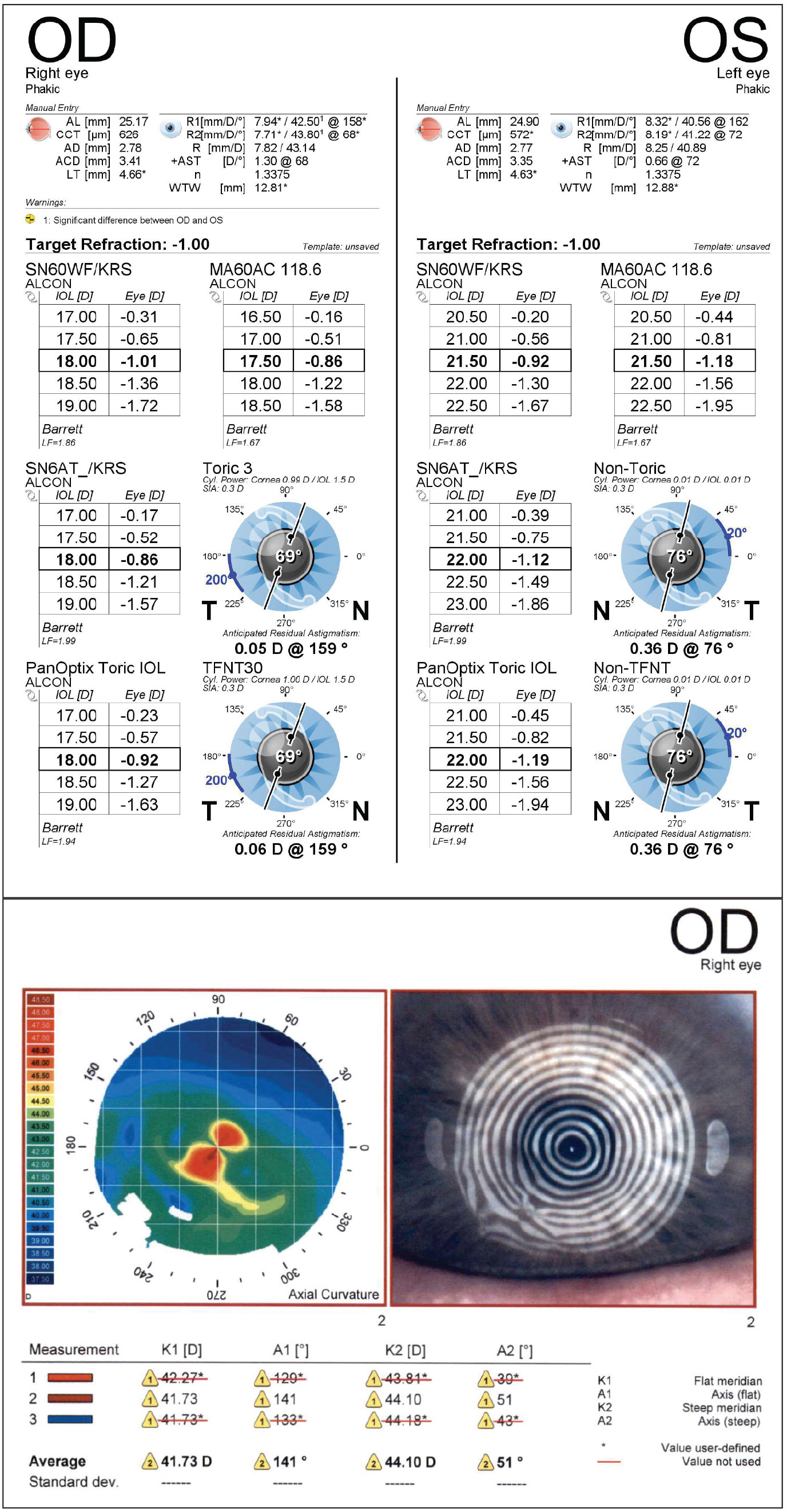
Figure 3 shows an example of the effect of epithelial edema on biometry and cataract surgery. This patient who presented for cataract and corneal transplant evaluation had a history of Fuchs’ dystrophy and primary open-angle glaucoma. His topography map showed a regular, bow-tie pattern of astigmatism and unremarkable biometry. He underwent a combined phaco-IOL-DMEK procedure with +18 D IOL, aimed at -1 D but ended up with a refraction of approximately +2.75 sph at one month.
On topography, the mires were in retrospect quite distorted, though the map showed regular astigmatism. Comparing the biometry of the right eye and left eye, which didn’t have epithelial edema, showed differing keratometry values, with the right eye measuring much steeper. Fortunately, once the cornea fully healed, the patient drifted back closer to plano. However, this is a cautionary tale that those pockets of epithelial edema really do affect biometry significantly.
 |
|
Figure 3. Epithelial edema can have a profound impact on biometry. Note the distorted mires despite a pattern of regular bow-tie astigmatism. |
Miotics
Pilocarpine and brimonidine are the two most commonly used miotics (e.g., Vuity 1.25% and Lumify 0.025%, respectively). Pilocarpine is both a cycloplegic and a miotic, and is much more potent than brimonidine. This drug induces a refractive change through the pinhole effect and by inducing ciliary muscle contraction.9 On the upside, this causes increased depth of focus and increased accommodation, but it also leads to poor night-time vision, restriction of peripheral vision and carries a risk of peripheral retinal traction and brow aches.
Ophthalmic Suspensions
Suspensions are a heterogeneous mixture of solid particles within a solvent. The two most prominent examples among glaucoma drugs are Azopt (brinzolamide 1%) and Simbrinza (brinzolamide 1%/brimonidine 0.2%).
Suspensions on the ocular surface can cause a temporary decrease in contrast sensitivity and increase in forward light scatter since the particles in suspension scatter light directed toward the ocular surface.10 Both of these factors have the effect of reducing visual quality.
A study assessing Azopt’s duration of effect found that reduced vision quality tends to linger for about five to 10 minutes after instillation.10 While five minutes isn’t long in the grand scheme of things, for an advanced glaucoma patient with already poor contrast sensitivity that’s being made worse by instilling drops three times a day, that five to 10 minutes can become quite significant. Anecdotally, we’ve had patients complain of blur for much longer than this after instilling the drops.
In summary, any drops on the eye can have refractive consequences. If there comes a time where you’re scratching your head about a refractive complaint in a glaucoma patient, a few things to consider are potentially switching to non-preserved agents, looking for morphologic changes in the cornea and asking yourself whether pupil size modulation or the formulation of the drops could be playing a role.
Dr. Sivaraman is medical director of the Cincinnati Eye Institute and serves as co-chair of the Clinical Governance Board. She has no related financial disclosures.
1. Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocul Pharmacol Ther 2014 Mar-Apr;30:2-3:163-9.
2. Datta S, Baudouin C, Brignole-Baudouin F, Denoyer A, Cortopassi GA. The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and
preferentially affects lhon mutant cells. Invest Ophthalmol Vis Sci 2017 Apr 1;58:4:2406-2412.
3. Van Bergen NJ, Crowston JG, Craig JE, Burdon KP, Kearns LS, Sharma S, Hewitt AW, Mackey DA, Trounce IA. Measurement of systemic mitochondrial function in advanced primary open-angle glaucoma and leber hereditary optic neuropathy. PLoS One 2015 Oct 23;10:10:e0140919.
4. Walsh K, Jones L. The use of preservatives in dry eye drops. Clin Ophthalmol 2019;13:1409-1425.
5. Koh S, Maeda N, Ikeda C, et al. Ocular forward light scattering and corneal backward light scattering in patients with dry eye. Invest Ophthalmol Vis Sci 2014;55:10:6601-6606.
6. Tran JA, Jurkunas UV, Yin J, Davies EC, Sola-Del Valle DA, Chen TC, Lin MM. Netarsudil-associated reticular corneal epithelial edema. Am J Ophthalmol Case Rep 2022 Jan 20;25:101287.
7. Chung D, Meier EJ. Rapid and reversible alteration in corneal contour and power associated with netarsudil/latanoprost. Am J Ophthalmol Case Rep 2022 Mar 25;26:101501.
8. Ganesh D, Coleman AL, Shibayama VP, Tseng VL. Netarsudil-induced corneal flattening in a child with secondary open-angle glaucoma. Case Rep Ophthalmol 2022 May 2;13:2:330-335.
9. Price FW Jr, Hom M, Moshirfar M, Evans D, Liu H, Penzner J, Robinson MR, Lee S, Wirta DL. Combinations of pilocarpine and oxymetazoline for the pharmacological treatment of presbyopia: Two randomized phase 2 studies. Ophthalmol Sci 2021 Oct 2;1:4:100065.
10. Hiraoka T, Daito M, Okamoto F, Kiuchi T, Oshika T. Contrast sensitivity and optical quality of the eye after instillation of timolol maleate gelforming solution and brinzolamide ophthalmic suspension. Ophthalmology 2010 Nov;117:11:2080-7.



