After being illegal in the United States for many years, cannabis has recently been legalized in many states. In fact, using marijuana for medical reasons is now legal in more than 25 states. For that reason, many patients now have ready access to cannabis, and they often have questions about whether it’s harmful or helpful for various medical conditions.
In addition, FDA-approved pharmaceuticals containing cannabis are now available that have been proven safe and effective in managing some very debilitating medical conditions. The FDA has approved cannabidiol (CBD)-containing products such as Epidiolex for severe forms of epilepsy such as Dravet Syndrome and Lennox-Gastout Syndrome. Dronabinol and Nabilone, which contain tetrahydrocannabinol (THC)—the compound in cannabis that has psychotropic effects—have been approved for nausea, vomiting and appetite stimulation chemotherapy-related cachexia. Researchers are also exploring the use of CBD to control other neurologic syndromes such as tics and Tourette’s.
Although our specialty is eye care, it’s not uncommon for ophthalmology patients to ask about how they might use cannabis to address a variety of problems in their own lives. The questions our patients most often ask relate to using cannabis to manage difficulty sleeping, pain and glaucoma.
Unfortunately, when it comes to glaucoma, there have been very few well-conducted studies of the effects cannabis has on intraocular pressure—much less other aspects of the disease. The reason such studies have been limited is that the FDA classifies cannabis as a Schedule I drug, similar to heroin, LSD and ecstacy. These drugs are considered likely to be abused and lacking in medical value, so there are strict controls on conducting any research involving them. As a result, very little clinical data on cannabis and glaucoma exists, so we haven’t been able to offer our patients many recommendations.
Nevertheless, it behooves us to educate ourselves about what we do know, because our patients are asking us about these issues. Here, I’ll share some of what we’ve learned about the cannabinoid compounds over the years, including what my own current research is revealing.
Cannabis and the Body
Many people talk about marijuana when they really should be discussing cannabis. Cannabis is a genus of flowering plants in the Cannabaceae family, which consists of three primary species: Cannabis sativa; Cannabis indica; and Cannabis ruderalis. The term marijuana has negative connotations; it’s used to refer to specific varieties of cannabis that contain more than 0.3 percent THC. CBD, on the other hand, has no psychotropic effects.
Cannabis contains multiple compounds—more than 480, of which about 65 have been identified as phytocannabinoids (including CBD and THC). Cannabis also contains about 120 compounds that give it its characteristic aroma—mainly volatile terpenes and sesquiterpenes. Not surprisingly, most patients don’t know much about cannabis; many don’t even understand the distinction between THC and CBD.
One of the main reasons cannabis is a topic of such interest is that the body produces its own cannabinoid chemicals that have physiological effects; they play key roles in regulating things such as memory, concentration, body movements, time awareness, appetite and pain. In fact, we have receptors for cannabinoids throughout our bodies—including in parts of the eye such as the ciliary body. As a result, we can take exogenous cannabinoids in the form of THC or CBD, and they’ll have an effect on us; our bodies are already wired to react to those chemicals.
The idea of using cannabinoids as medicine actually started in ophthalmology, back in the 1970s. A patient who had pigmentary glaucoma noticed the characteristic halos around lights that he’d see when his IOP went up. He also noticed that those halos disappeared when he smoked marijuana. So, he petitioned the court to be able to obtain and use marijuana for his glaucoma. He was granted that permission, making him the first legal user of cannabis for medical reasons.
This prompted interest in researching the use of cannabis to treat glaucoma, but because cannabis is a Schedule I drug, you have to go through various governmental entities to get permission to proceed. In particular, in order to perform research involving cannabis, you have to demonstrate the scientific validity and ethical soundness of your study to the National Institute for Drug Abuse, which exists to conduct and support scientific research on drugs and drug abuse, and to advise the public and the government. (It contracts with the University of Mississippi to grow cannabis for use in research studies.) Today, those obstacles to research still persist, because cannabis is still in the same category as heroin and LSD.
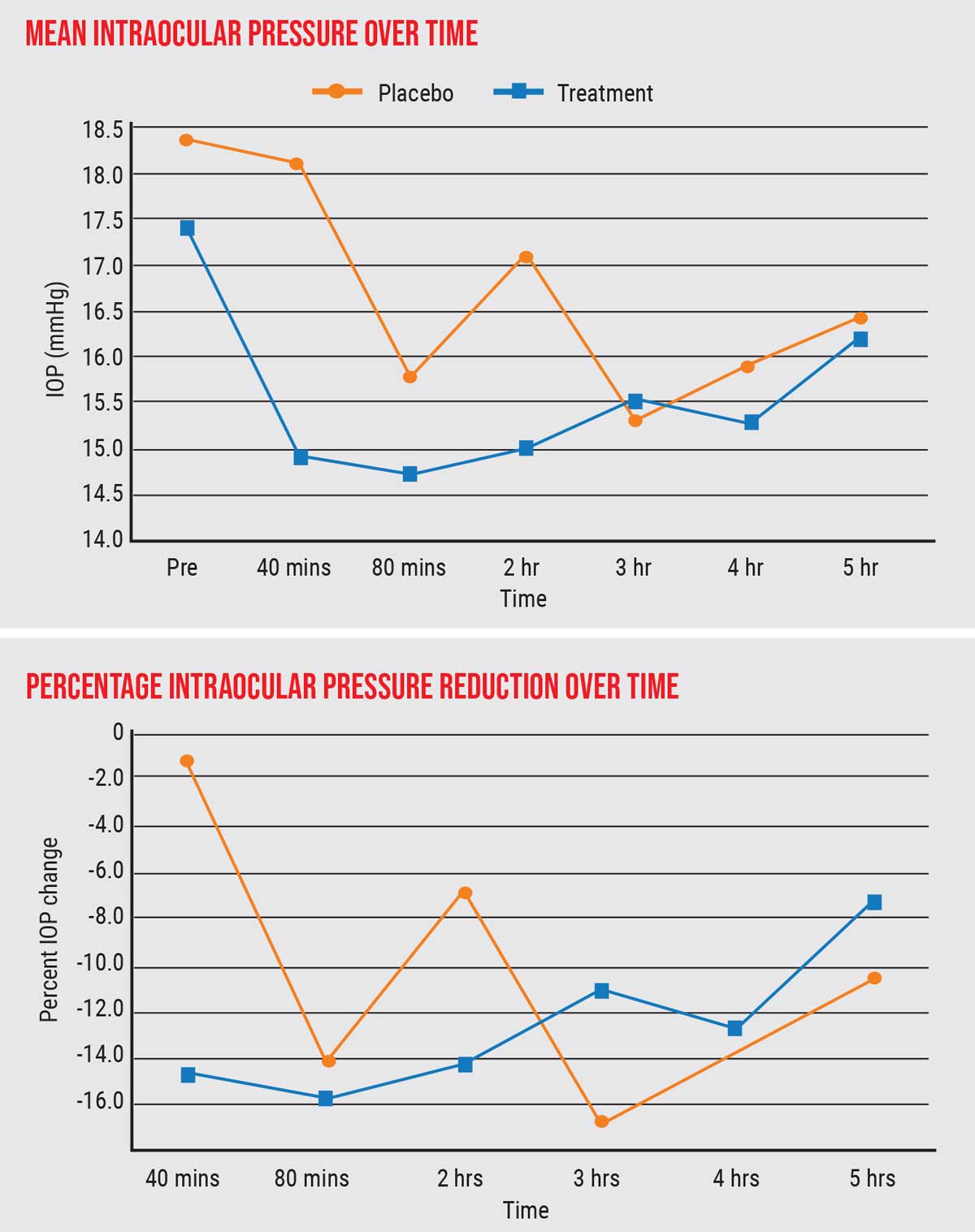 |
| One of the author's studies found a substantial and significant decrease in IOP in subjects smoking cigarettes with THC, compared to placebo. The patients went from a mean IOP of 17.5 mmHg prior to smoking down to lower than 15 mmHg, 15 percent below baseline. Click image to enlarge. |
What the Limited Data Show
Early studies in the 1970s and 80s were very small-scale and not controlled, and they involved only a handful of healthy volunteers, not glaucoma patients.1-6 Those studies basically found that cannabis can lower eye pressure by about 25 percent for two to four hours in 60 to 65 percent of individuals. In the early 1980s there were also a few poorly controlled studies looking at topical THC drops, which showed no effect on IOP. As far as systemically administered cannabis, such as ingested, injected or sublingual cannabis, there’s very limited data.2
Currently, many patients are asking about the use of CBD in particular, because they can get it at just about any grocery store in the form of oils, tinctures, creams, tablets and gummy bears. Very few studies of the effect of CBD on glaucoma in humans have been conducted, but those few small-scale studies have found no IOP-lowering effects from CBD in low doses. In fact, some sublingual studies found transient IOP elevation with higher doses.2
That finding parallels a larger body of animal evidence. Data from some rat and mouse studies looking at CBD and THC suggest that while THC may act as an agonist of the cannabinoid receptors in the eye, CBD can act as an antagonist of those receptors.4 In short, the larger body of evidence suggests that CBD is not an effective way to lower eye pressure.
In contrast, the existing studies have provided evidence that unlike CBD, THC does lower IOP (the ineffectiveness of topical drops notwithstanding). We know that cannabinoid receptors are located throughout the ciliary body, the ciliary muscle and epithelium, and the trabecular meshwork, so these may be the sites through which THC affects eye pressure. In addition, THC is known to have hemodynamic effects; it may lower episcleral venous pressure. Furthermore, a few animal studies suggest that it may also have potential neuroprotective effects. Right now we just don’t know for sure. (Some animal studies also found a difference in IOP-lowering related to gender,7 but that hasn’t been found in human studies.)
Because of the Schedule I status and the stigma associated with it, all research on cannabis basically ceased in the 1980s; it was just too difficult to get around the regulations. Among other things, limited high-quality data has impacted the current American Academy of Ophthalmology and American Glaucoma Society positions on the use of cannabis to treat glaucoma. They don’t support it, largely because there’s too little information to justify such support.
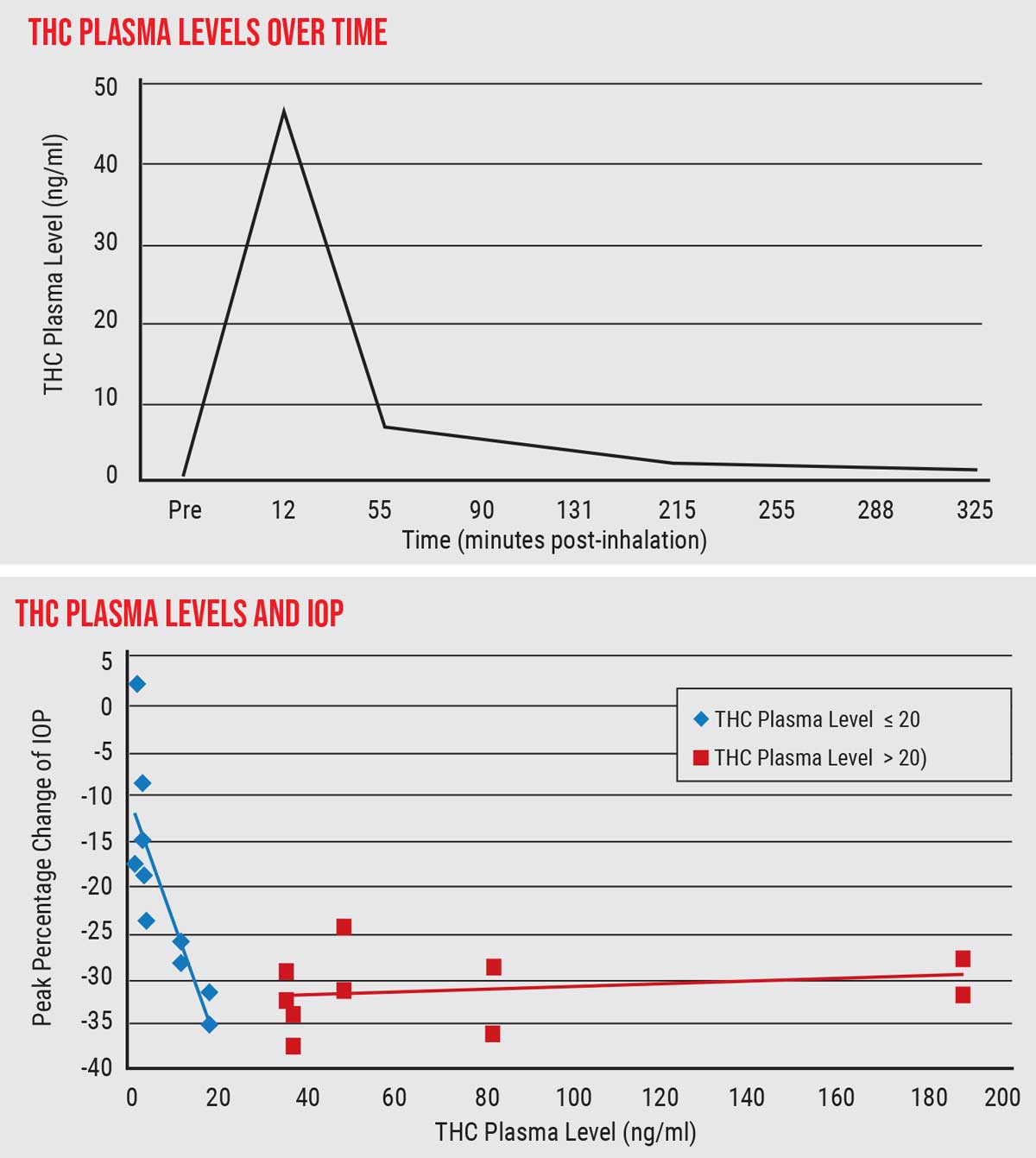 |
| THC is metabolized quickly, soon disappearing from the bloodstream. (Top graph) Decline in IOP paralleled rising THC plasma levels up to 20 ng/ml; above that, IOP did not decline. (Bottom graph) This suggests that a limited intake of THC—possibly a small enough amount to avoid psychotropic effects—could accomplish significant IOP lowering. Click image to enlarge. |
Conducting Our Own Study
Our group decided to perform the first randomized, double-blind, placebo-controlled, prospective study looking at the effect of inhaled active THC compared to placebo.8 After obtaining IRB approval to conduct the project, we enrolled healthy adult subjects without a history of eye disease, as part of a much larger study being done for the California Highway Patrol. The larger study was investigating reaction times and driving performance after cannabis inhalation, to determine at what dose people become impaired; our study was a small sub-study looking at the ocular effects of THC on IOP.
For our study, patients were given 700-mg cigarettes, either a placebo containing terpenes—so they smelled exactly like the cannabis cigarettes—or containing either 5.9-percent or 13.4-percent THC. The testing took place in negative-pressure rooms; the subjects were allowed to smoke until they felt high. Blood plasma levels were checked 30 minutes after inhalation; IOP and blood pressure were assessed every 30 minutes for four hours.
Among the results of our study:
- We found a substantial and significant decrease in IOP in subjects smoking cigarettes with THC compared to placebo. The patients went from an average IOP of 17.5 mmHg prior to smoking, down to lower than 15 mmHg, 15 percent lower than baseline. A 15-percent reduction, when you start out with normal pressure, is quite significant—on a par with what you’d see with a single-agent IOP-lowering eye drop.
- The lower pressure was sustained for up to three hours.
- In terms of systolic and diastolic blood pressure, we found no statistically significant differences between the placebo group and cannabis group. There were some differences, as the graphs show (graph below), but the differences were only statistically significant at a single time point (marked with an asterisk).
- We confirmed that THC is metabolized very quickly; it gets absorbed into tissues and disappears from the bloodstream very quickly.
- There was a linear correlation between THC level in the blood plasma and IOP reduction, up to about 20 ng/ml of THC. Additional elevation of plasma THC, however, didn’t correlate with further IOP lowering. (See graph above.) In other words, achieving 20 ng/ml of blood plasma level of THC was all that was required to achieve the maximum IOP-lowering effect.
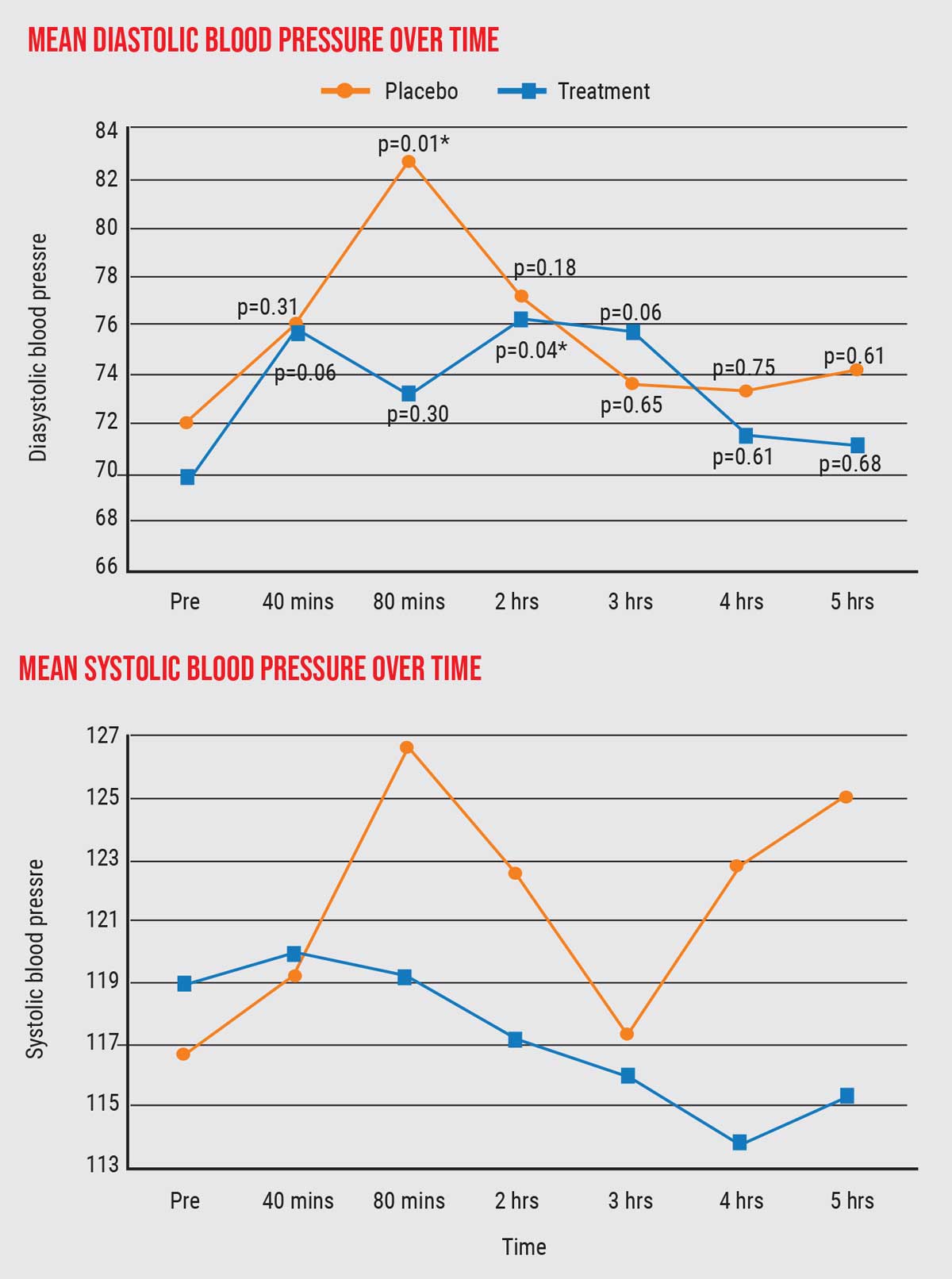 |
| The data revealed only one point of statistically significant difference between the placebo group and cannabis group in diastolic or systolic blood pressure (asterisk). Click image to enlarge. |
This last finding is particularly interesting, because some people felt high at a blood plasma level of 20 ng/ml, while others did not. This suggests that, for at least some people, it may be possible to maximize the pressure-lowering effect without requiring sufficient THC to have a psychotropic effect. In other words, you may not need to get high to get the IOP-lowering you want.
That’s important because an obvious potential drawback to treating glaucoma with THC has always been the possibility that a patient would need to be high all day to control eye pressure. Our data suggests that this may not be the case. Of course, this would depend on an individual’s tolerance for THC, but 20 ng/ml is a much lower plasma level than what was thought would be required to achieve substantial IOP lowering.
What’s next?
As you can see, there’s a growing body of evidence confirming the IOP-lowering effects of cannabis. Of course, there are still concerns about the psychotropic effects of THC and its limited duration of action. However, plenty of evidence suggests that it’s a powerful IOP-lowering compound. So, we need to do more investigation to see where and how it might benefit patients, and in what setting.
As noted, the subjects in our study, like those in previous studies, were healthy individuals without elevated IOP at baseline. That raises the question: Would the same effect appear in a patient who has an elevated pressure at baseline? In most scenarios involving pressure-lowering treatments, the higher your starting pressure, the more profound the IOP reduction you typically achieve. However, that’s based on prior studies that have been done using different glaucoma medications and surgical interventions; we can’t assume it would be true in this case. So, the next step will be to try this on patients with glaucoma or ocular hypertension, as well as investigating the hemodynamic and vascular effects inside the eye. We’re now conducting those studies.
Ideally, in order to make cannabis feasible as a treatment for glaucoma, it should lower pressure for 24 hours—not just three hours. Or, it should at least lower it throughout the nocturnal period, since IOP seems to go up at night when we’re sleeping. (This might also be a reasonable time to use a treatment that can cause drowsiness or mental status changes.) To mitigate the limited duration of action, a number of different sustained-delivery mechanisms might be employed, but those possibilities have yet to be tested.
Topical cannabis drops have so far not been useful, due to poor penetration through the cornea. However, some companies are investigating potential ways to improve its penetration. Placing a depot inside the eye is also a possibility, but those are very difficult to get approved because of all the potential issues to consider, such as endothelial cell loss and damage to intraocular structures. Topical drops are a far easier solution—if they can be made to work.
In terms of future research:
- We need to conduct 24-hour studies on not just inhaled cannabis, but ingested and other systemically delivered forms of THC as well.
- We need to figure out what the optimal dosing regimen is, keeping in mind our discovery that we may not need to get people high in order to get their pressure down.
- We need to develop improved topical application strategies.
- We need to look carefully for potential gender differences in efficacy (found in some animal studies).
Another issue that’s less obvious is that cannabis’s effect on ocular perfusion is an important part of the equation. If the drug lowers IOP but also lowers ocular perfusion, that will counteract the benefits of lowering the IOP. In other words, if it turns out that one of the hemodynamic effects of cannabis is to lower blood flow to the optic nerve, the drop in pressure won’t be beneficial. That’s one of the subjects of our ongoing research.
Talking to Your Patients
Because cannabis research has been so highly stigmatized, people haven’t done serious studies for decades. There are so many barriers to high-level investigations in place that most researchers decide it’s not worth going after. But I think there’s merit to looking at it, so we’re making the effort.
Meanwhile, your patients are likely to be curious about this topic. So: What should you tell them? I tell my patients that we know that cannabis does lower IOP for a few hours, but we don’t know what it’s doing to ocular blood flow, which is equally important. That means that at this time we really don’t know whether cannabis is helpful or harmful for glaucoma, and therefore we can’t comment on dosage recommendations. I also tell patients that CBD doesn’t help with eye pressure.
Finally, I tell my patients that until we have more information on cannabis, if they wish to employ lifestyle changes to assist with their glaucoma management, options like stress reduction, aerobic activity, limitation of caffeine and mindful meditation are safe places to start.
This article has no commercial sponsorship.
Dr. Mosaed is a professor of ophthalmology and director of the Glaucoma Division of the Gavin Herbert Eye Institute at UC Irvine. She is a consultant to Aerie and Bausch + Lomb. Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
1. Hepler RS, Frank IR. Marijuana smoking and intraocular pressure. JAMA 1971;217:10:1392.
2. Tomida I, Azuara-Blanco A, House H, et al. Effect of sublingual application of cannabinoids on intraocular pressure: A pilot study. J Glaucoma 2006;15:5:349-53.
3. Cooler P, Gregg JM. Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. South Med J 1977;70:951–4.
4. Green K, Roth M. Ocular effects of topical administration of delta 9-tetrahydrocannabinol in man. Arch Ophthalmol 1982;100:265–7.
5. Jay WM, Green K. Multiple-drop study of topically applied 1% delta 9-tetrahydrocannabinol in human eyes. Arch Ophthalmol 1983;101:591–3.
6. Chiang CW, Barnett G. Marijuana effect and delta-9-tetrahydrocannabinol plasma level. Clin Pharmacol Ther 1984;36:234–8.
7. Miller S, Daily L, Leishman E, et al. Δ9-Tetrahydrocannabinol and cannabidiol differentially regulate intraocular pressure. Invest Ophthalmol Vis Sci 2018;59:15:5904-5911.
8. Mosaed S, Smith AK, Liu JHK, et al. The relationship between plasma tetrahydrocannabinol levels and intraocular pressure in healthy adult subjects. Front Med (Lausanne) 2022;8:736792.