Intraocular pressure control is the mainstay of glaucoma treatment, but the disease is multifactorial, and many patients experience progression despite reduced pressures. Many patients also simply wonder what else they can do for their glaucoma. While the dearth of evidence for most alternative therapies may make clinicians hesitant to recommend them to their patients, experts also acknowledge that there may be seeds for future therapeutics in some of them. “Ophthalmologists always need to be looking for new treatments, because our patients need better interventions than what we have now,” points out Catherine M. Marando, MD, of the Massachusetts Eye and Ear Infirmary Glaucoma Service.
Here, we’ll look at the current glaucoma drugs, what’s coming down the pipeline and examine the evidence for some alternative therapies.
Standard-of-Care Approaches
After grading glaucoma severity and setting a target IOP goal, clinicians typically initiate medical therapy. “My approach to glaucoma treatment usually includes topical glaucoma therapy, laser trabeculoplasty, minimally invasive angle-based procedures, filtering surgery and tube shunt surgeries,” says Teresa C. Chen, MD, an associate professor of ophthalmology at Harvard Medical School, Massachusetts Eye and Ear Infirmary. “The final treatment plan usually depends on patient preference, patient age, glaucoma staging and tissue quality.”
Prostaglandin analogs are a common first-line therapy for most patients because they’re effective, safe and dosed once-a-day, says Albert S. Khouri, MD, director of the glaucoma service at Rutgers New Jersey Medical School. “This landscape has shifted recently with newer medications such as latanoprostene bunod and the fixed-combination latanoprost-netarsudil, which have been shown to be slightly more effective than a prostaglandin alone in clinical trials,” he says.1,2
“What’s also changed over the last five years or so is the use of lasers earlier in the treatment paradigm,” he continues. “I offer to do selective laser trabeculoplasty as a first-line treatment for patients. In my experience, younger patients who are working and who may struggle with adhering to a medication regimen or may not want their eyes to be red from topical therapy are more likely to accept laser treatment as their first option.”
“I always initially offer patients either SLT or medical therapy,” says Joel S. Schuman, MD, co-director of the glaucoma service at Wills Eye Hospital in Philadelphia. “About 60 percent choose medical therapy as their initial first-line treatment. I generally start off with a prostaglandin analog if the patient is comfortable with the side effects: changes in eye color and pigmentation around the eyes; thicker, darker eyelashes; and potential orbital fat atrophy. Individuals with hazel eyes are at the highest risk for these changes while those with ice blue eyes have less risk of iris color change. If the patient is comfortable with the possibility of permanent eye-color change then we go ahead with prostaglandin analogs as the initial medical therapy.”
If first-line medical therapy or SLT isn’t sufficient to meet the target IOP reduction, a second medical agent is added. “I generally go with a beta blocker—timolol, specifically, assuming the patient doesn’t have contraindications for using beta blockers such as asthma or bradycardia,” Dr. Schuman says. “Then as a third-line agent, I might add either brimonidine or dorzolamide as a combo drop with timolol. The choice will be based on the patient’s insurance. Many insurers for the patients I [saw when I practiced] in New York City wouldn’t pay for brimonidine-timolol (Combigan) but will pay for dorzolamide-timolol (Cosopt).
“For a fourth-line agent, I’ll often add netarsudil (Rhopressa) or latanoprost-netarsudil (Rocklatan), depending on insurance coverage,” he says. “Fifth-line may include brimonidine or dorzolamide, whichever one the patient isn’t already taking. For patients who are very reluctant to have surgery, I’ll even add pilocarpine. If one of the medications isn’t effective in that individual, I’ll stop that drug to avoid piling on more.”
Juggling multiple medications is often difficult for patients, and the cumulative effect of preserved glaucoma drops often leads to ocular surface issues such as irritation and dry-eye disease. Dr. Khouri says that what clinicians consider “maximal tolerated medical therapy” isn’t the same as it once was. “This used to mean multiple bottles of medicine years ago, but now with more effective fixed-combination medications, maximal medical therapy may mean two bottles,” he explains. “Two fixed-combination bottles such as latanoprost-netarsudil or dorzolamide-timolol could be maximal therapy at three drops per day.”
New Treatments
In addition to fixed-combination drops, sustained-release versions of currently used glaucoma medications such as Durysta (Allergan) may help reduce the drop burden. Glaucoma specialists say they’d like to see future reusable options and those that provide controlled release over many years. There are several candidates in the pipeline, including TravoprostXR (EMV-515; Alcon/Aerie), iDose TR travoprost implant (Glaukos), the OTX-TIC travoprost intracameral implant (Ocular Therapeutix) and an IOL with drug-eluting pads attached to the haptics (SpyGlass Pharma).
Santen’s Omlonti (omidenepag isopropyl ophthalmic solution) 0.002% recently received FDA approval in September 2022, giving patients another potential first-line medical option. Omlonti is a relatively selective prostaglandin EP2 receptor agonist that increases aqueous humor drainage through the trabecular and uveoscleral outflow pathways. In a U.S. Phase III study, Omlonti was noninferior to timolol, resulting in IOP reductions ranging from 5 to 7 mmHg from an average baseline IOP of 24 to 26 mmHg, compared with timolol’s 5- to 7-mmHg IOP reduction and latanoprost’s 6- to 8-mmHg reduction.3
“In many ways Omlonti works similarly to our current prostaglandin analogs; ours are FP receptor agonists, and this works on a different receptor,” says Dr. Schuman. “It has some of the same side effects, including iris color change. Omlonti might work in patients for whom our current prostaglandin analogs aren’t adequate.”
The first once-daily brimonidine may be around the corner with Visiox Pharma’s PDP-716 (brimonidine tartrate 0.35%). The New Drug Application was accepted by the FDA in December 2022. PDP-716 uses TearAct fine resin technology, which the company says provides consistent, sustained drug release and IOP control.
“Having a once-a-day brimonidine would be great,” says Dr. Schuman. “It’s unclear whether the increased time on the eye will increase the allergic response we sometimes see with brimonidine, but a once-a-day drop is certainly preferable to two or three drops per day.”
The Phase III study (NCT03450629) included 682 participants, randomized to receive either PDP-716 or three-times daily Alphagan-P (brimonidine tartrate 0.1%). The two drugs demonstrated functionally equivalent IOP reduction across all nine timepoints. Treatment-emergent adverse events were similar, with a 38.8-percent rate in the PDP-716 group and 33.2-percent rate with Alphagan-P.4
Alternative Approaches
Clinicians may be surprised by the number of patients using alternative therapies to treat their glaucoma today. It’s been estimated that between 5 and 11 percent5,6 of patients with glaucoma use some form of complementary and alternative medicine. However, experts say it’s rarely talked about in the clinic and patients are unlikely to share this information.
Dr. Marando, who co-authored a review of the evidence for complementary glaucoma medicine with Dr. Chen, says she was surprised to find in the study that almost half of glaucoma patients (i.e. 42 percent) in the United States have tried at least one complementary alternative medication, and that most didn’t disclose this to their physician.
 |
| Ginkgo biloba has demonstrated transient improvements in visual field indices, but its antithrombotic properties may lead to adverse events such as retinal hemorrhage and hyphema. (Courtesy Getty Images) |
“This suggests that perhaps I should be more proactive in asking my patients if they are using marijuana, Ginkgo biloba extract, bilberry fruit extract or acupuncture,” she says. “In addition, there was a high number of poor-quality studies (small sample sizes, short duration of follow up, etc.) that drew major conclusions about the efficacy of an intervention. I would hate for these findings to be misinterpreted by patients, who may not have the training and experience to critically evaluate medical literature.”
According to Dr. Marando, most patients discover alternative therapies through a “non-health care provider.” She says, “As a result, these interventions aren’t regulated or prescribed in an evidence-based manner, and I suspect there’s little regard for potential serious side effects. I’m not aware of interactions of alternative therapies with glaucoma drops, however this doesn’t mean it isn’t possible, given the lack of meaningful data. Patients need to be aware that alternative treatments aren’t harmless, and that there are potential negative side effects associated with their use.”
Many of the remedies touted for glaucoma have an effect on the vascular system. “Some can cause coagulopathy,” Dr. Khouri points out. “This is an example of why it’s important for clinicians to inquire about supplements, particularly if you’re taking the patient to the operating room. If the patient is on some alternative therapy that has a blood thinning effect, this could increase the patient’s risk of bleeding, not just in the operating room but also after the glaucoma surgery. There’s the risk of intraocular bleeding, including a suprachoroidal hemorrhage, which can be devastating in terms of its effect on vision. Asking about alternative therapy use can help reduce the risks of surgery.”
Dr. Khouri says he thinks there’s some potential in alternative medicine for glaucoma. “Challenges remain, and the timeline on these studies is long,” he says. “It can take decades to go from Phase I to Phase II to Phase III, and these studies are so costly that unless the Phase I and II studies show significant promise, it’s unlikely further studies will occur with these alternative treatments.”
The literature on complementary and alternative medicine suggests possible short-term benefits for some commonly used remedies but overall weak evidence for their use to treat glaucoma.7 Dr. Chen says that when it comes to alternative glaucoma therapies, she doesn’t bring up the topic unless the patient asks her about it. “When they do bring it up, I tell them that the benefits are transient and theoretical, and the risks are rare but real,” she explains. “I explain that alternative therapies aren’t an advisable treatment option and may in fact delay use of proven therapies, thus causing the patient to lose vision irreversibly during this time.”
“All of these supplements are just that—supplements,” agrees Dr. Schuman. “I don’t recommend them if a patient’s IOP isn’t controlled. IOP control is our primary aim because there’s excellent evidence that not controlling IOP adequately ultimately leads to deterioration of the optic nerve and visual function in glaucoma. If a patient wants to adjunctively take supplements and is consistently controlling their IOP, I think that’s fine, but taking supplements instead of controlling IOP is not fine.
“That said, I’m a scientist and a clinician, and I do believe there’s a lot we don’t know,” he continues. “It’s important for us to keep an open mind. It’s easy to dismiss all supplements out of hand, but some evidence exists, and it’s important to review all safe options with your patient if there’s a potential that those interventions will be beneficial.
“There’s limited evidence on a number of nutraceuticals or supplements,” he continues. “The best evidence now seems to be for three compounds in particular: nicotinamide, especially combined with pyruvate, and acetylcholine. When I have patients who are looking for something beyond conventional therapy, especially those who are continuing to progress despite single-digit or low-teen pressures, I’ll review these with them. I have a handout that covers the compounds and dosages.”
Nicotinamide and Pyruvate
Developing neuroprotective and neuroenhancement therapies for glaucoma patients is an increasingly important focus of research, especially for patients who continue to progress despite IOP lowering measures. Neuroprotection focuses on preventing retinal ganglion cell death while neuroenhancement aims to improve the function of retinal ganglion cells that are damaged but not yet dead.8
Nicotinamide is a form of vitamin B3 that the body makes from niacin-rich foods such as eggs, legumes, green vegetables, nuts and fish. This dietary supplement is available over the counter and is sometimes used for skin conditions, niacin deficiencies and as a preventative measure against skin cancer.9
Nicotinamide is a precursor to nicotinamide adenine dinucleotide (NAD), a molecule that plays a key role in energy and redox metabolism, but that decreases with age. NAD of the retina and optic nerve declines as a function of IOP, according a study using a rat model of ocular hypertension,10 and has been demonstrated to be reduced in the sera of patients with POAG.11
Pyruvate is formed during glycolysis and plays a critical role in energy production pathways in the body. Studies in rats and mice have reported IOP-mediated decreases in retinal pyruvate levels. RNA sequencing showed that gene expression changes impact pathways mediating metabolism and transport of glucose and pyruvate, but oral supplementation of pyruvate was neuroprotective in both rats and mice.12
The results of a Phase II randomized clinical trial for nicotinamide and pyruvate for neuroenhancement in open-angle glaucoma, published in JAMA Ophthalmology in 2021, reported that this combination conferred significant short-term visual function improvement.13 The researchers hypothesized that the combination of nicotinamide and pyruvate could improve retinal ganglion cell function in patients with glaucoma. Participants in the Phase II trial were randomized to high oral doses of nicotinamide (1,000 to 3,000 mg) and pyruvate (1,500 to 3,000 mg) or placebo. A total of 32 participants (mean age 64.6 years) completed the study (21 intervention, 11 placebo) with a mean follow-up of 2.2 months. No adverse events were reported.
The primary endpoint was number of visual field test locations improving beyond normal variability in the study eye. In the study, this number was significantly higher in the intervention group than the placebo group (median IQR 16 vs. 7; p=0.005). The pattern standard deviation rates of change for visual field global indices suggested improvement with the intervention compared with the placebo (median -0.06 vs. 0.02 dB/week; 95% CI 0.02 to 0.24; p=0.02) but the mean deviation (0.04 vs. -0.002 dB/wk; 95% CI -0.27 to 0.09; p=0.35) and visual field index (0.09 vs. -0.02 percent per week; 95% CI -0.53 to 0.36; p=0.71) did not. Some patients (30 percent) reported mild gastrointestinal discomfort due to the high vitamin doses.
Vitamin B3 supplementation is likely safe based on other clinical trials for diseases. More studies are needed to confirm this combination’s benefits for slowing visual field progression or providing sustained functional improvement over extended periods, but the researchers aver that targeting the same pathways may lead to the development of new neuroprotective therapies. A two-year randomized multicenter clinical trial of vitamin B3 with an enrollment aim of 1,800 participants is currently ongoing in Australia, Singapore, Sweden and the UK.
Acetylcholine
Acetylcholine is a neurotransmitter and neuromodulator. As a food supplement, acetylcholine may help control blood pressure. Eating acetylcholine-rich foods such as eggplant and shiitake mushrooms can raise levels of this nutrient in the body.
In stressed hypertensive individuals, taking eggplant powder (1.2 g/day; 2.3 mg of ACh/day for 12 weeks) was shown to reduce blood pressure and improve psychological stress in a randomized placebo-controlled study of 100 participants.14 Participants with normal-high blood pressure had decreased hospital diastolic blood pressure at week eight and those with grade-1 hypertension had decreased systolic and diastolic blood pressure at week 12 compared with the placebo group. The researchers estimated that the functional cause was acetylcholine.
Acetylcholine, released from starburst amacrine cells in the retina, has been suggested to provide neuroprotection to the retinal ganglion cells that are lost, overwhelmed or compromised under glaucomatous conditions.15 In vivo rat glaucoma models indicate that PNU-282987, an α7 nicotinic acetylcholine receptor agonist, could significantly reduce glaucoma-associated retinal ganglion cell loss and may be a potential therapeutic target for glaucoma treatment.15 In the study, episcleral veinous NaCl injections used to induce glaucoma caused significant cell loss (mean loss 27.35 percent) in the retinal ganglion cell layer at one month. Retinal ganglion cell loss was eliminated if 5 µL of 100 µM PNU-282987 was injected into the eye an hour prior to the NaCl injection. Since PNU-282987 was applied before inducing glaucoma, this potential treatment would act as a preventative measure for patients at high risk for glaucoma.
Cannabis
“One of the most common alternative treatment questions I get from patients is about cannabis use,” Dr. Khouri says. “We investigated public perception of marijuana use for glaucoma treatment in a study recently and found there’s a significant gap between patient and physician perceptions.16
“We looked at Twitter and analyzed tweets over the last two years,” he says. “The vast majority were in favor of cannabis use for glaucoma (72 percent, n=503) while 18 percent were opposed (n=124). Most tweets in favor of using cannabis came from individual Twitter users while those not in favor of cannabis came from accounts such as health-care media, ophthalmologists and other professionals. We need better public education on the role of cannabis in glaucoma treatment.
“If you examine the literature on cannabis and glaucoma, it shows short-lived effects on IOP—typically a duration of only a few hours,” he explains. “The fact that cannabis could lower eye pressure may seem favorable to patients but there are ill effects from smoke inhalation, not to mention the glaucoma-specific effect of fluctuating IOP. The IOP will go up once the cannabis effect wears off. We know from multiple clinical trials that all those ups and downs in pressure can have a deleterious effect on glaucoma progression, particularly if the disease is severe. In early glaucoma it may be less relevant—we don’t have sufficient data yet—but in severe glaucoma, the nerve is more susceptible to IOP fluctuations.”
Tetrahydrocannabinol
Tetrahydrocannabinol is a main psychoactive component of cannabis. The IOP-lowering mechanism of cannabis isn’t fully understood but researchers are exploring ways that isolated cannabinoids or synthetic analogs could produce sustained effects with fewer adverse side effects. Decades ago, THC studies were underway for glaucoma, but several challenges arose with creating a topical treatment targeting cannabinoid receptors. Dr. Marando says it was “fraught with issues, such as creating an adequate hydrophobic delivery system that’s well-tolerated and whether the therapy has any meaningful effect in humans.”
“The THC studies back then didn’t really pan out, but the initial work on a new THC-based drug seems promising,” Dr. Schuman says.
Skye Bioscience is developing a synthetic cannabinoid derivative to treat glaucoma. SBI-100 ophthalmic emulsion is a synthetic THC derivative molecule combined with the company’s nanoemulsion formulation that facilitates topical administration and penetration into the eye. In the single-ascending-dose arm of the Phase I study conducted in Australia, 18 of 24 participants were dosed with topical SBI-100 in concentrations of 0.5%, 1% and 2%, with no adverse events and expected mild to moderate adverse events. A multiple-ascending-dose arm was enrolled in April. Participants will be administered a topical dose of SBI-100 or placebo twice daily for five days.17
Herbal Medicine
Herbal remedies and supplements reportedly used for glaucoma include Ginkgo biloba, bilberry fruit extract, cannabis, turmeric/curcumin, coenzyme Q10,18 resveratrol, Tripterygium wilfordii (“thunder god vine”) and Lycium barbarum (goji berry).19
“Ginkgo biloba and bilberry are pretty popular among patients,” Dr. Khouri says. “These are the ones that commonly pop up if patients do a simple Google search on glaucoma and homeopathic medicine or alternative medicine. There’s data on Gingko biloba and bilberry being useful for glaucoma, as they are believed to play antioxidant and anti-inflammatory roles and reduce free radicals. Both also have some effects on the vascular system, a vasodilatory effect and anti-platelet function that could in theory be helpful for a patient with glaucoma to improve blood flow to the optic nerve head. However, there’s no consensus in the literature for whether this changes outcomes for glaucoma patients or not.”
Ginkgo biloba extract is derived from ginkgo tree leaves, which contain flavonoids and terpenoids and various bioactive compounds. Ginkgo has demonstrated antioxidant properties and short-term improvement in visual field indices, but it also has antithrombotic properties that may produce adverse ocular effects such as retinal hemorrhage and hyphema.20
Bilberry fruit (Vaccinium myrtillus) or European blueberry contains the flavonoid anthocyanin and is proposed to confer neuroprotective and anti-inflammatory effects. Adverse effects may include cachexia, anemia and icterus in the event of overdose.20
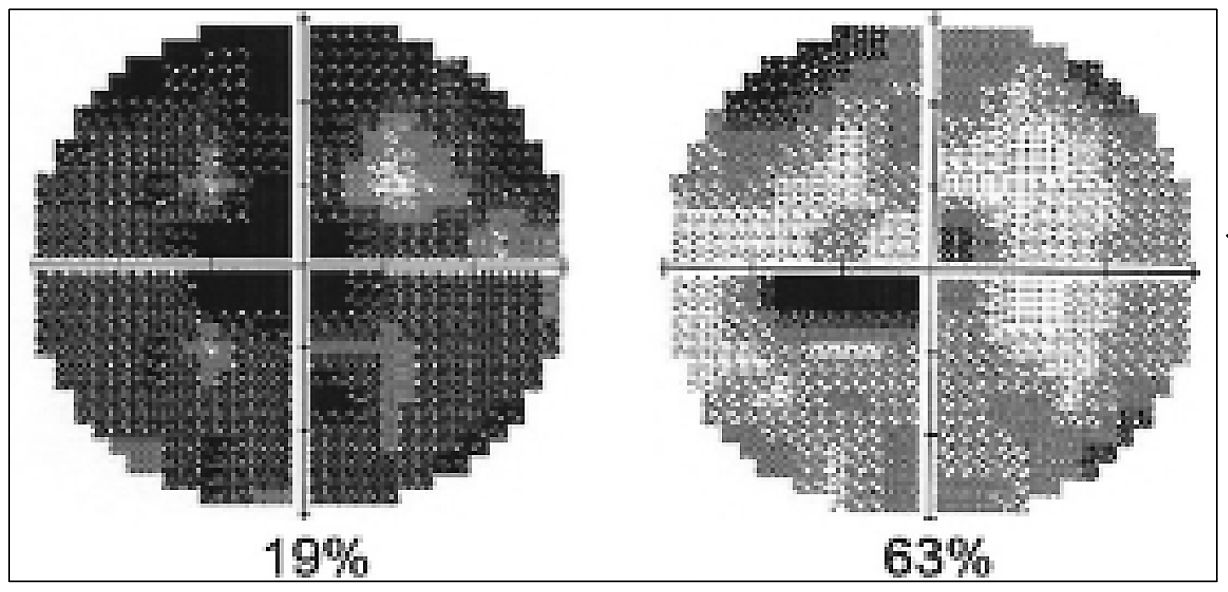 |
| Figure 1. A female glaucoma patient’s visual fields before and after a second treatment with repetitive transorbital alternating current stimulation. (Courtesy Bernhard Sabel, PhD) |
Neurostimulation
Neurostimulation therapies aim to reactivate malfunctioning retinal ganglion cells otherwise perceived as dead by applying electrical currents to relax muscles in the head and improve blood flow. Proponents say this approach can result in visual field improvements in certain patients, provided there are living cells to target.
“We call these surviving cells ‘silent neurons,’ ” explains Bernhard Sabel, PhD, a medical psychology professor at the Otto-von-Guericke-University Magdeburg in Germany and pioneer of vision restoration and the residual vision activation theory. “ ‘Silent neurons’ remain in a hibernation state caused by damage or continuous mental stress but can be reactivated by electrical stimulation, so they can send visual signals once more. The electrical pulses have a ‘double-punch’ effect because on one hand they mimic the electric pulses that brain cells use to communicate with one another, a kind of ‘wake-up call,’ and on the other hand they simultaneously enhance blood flow by vasodilation.”
Dr. Sabel, who has treated more than 2,000 patients over the last decade using microcurrent therapy at the Savir-Center in Magdeburg, Germany, says the therapy produces greater effects in those with advanced disease. “Patients who have very little vision loss won’t see major improvements because the improvement asymptotes and reaches the ceiling, while those with moderate or severe disease benefit more.”
A randomized clinical trial demonstrated a mean visual field improvement of 24 percent (n=45; mean age 59) with repetitive transorbital alternating current stimulation (rtACS) lasting for at least two months, compared with the 2.5-percent improvement observed in the sham stimulation group (n=37).21 Secondary analyses showed improved reaction times, improved near-threshold visual fields in the central 5 degrees and increased static perimetry thresholds after treatment, but no visual acuity changes compared with sham. The treatment stimulated the eye and optic nerve as well as the frontal cortex and subcortical regions of the brain inducing brain plasticity by way of improving functional brain network synchronization.22,23
Subsequent studies by his group on the role of brain plasticity in visual function22 point out indirect influential factors such as intracerebral pressure, eye movement, top-down modulation (cognition, attention) and the release of emotionally triggered stress hormones contributing to blood vessel dysregulation.24
“Mental stress has a negative influence on the development of glaucoma and on electrical stimulation therapy,” Dr. Sabel says. “We believe the main effect of electrical stimulation is the relaxation of the muscles that surround the blood vessels. To that end, we also incorporate eye movement exercises, massage and meditation to reduce stress and improve blood supply to the nerve cells involved in vision, reactivating the ‘silent’ neurons.
“There’s great variability in response to neurostimulation,” he continues. “Our experience today with the patients we treat for 10 days at our center shows that about 85 percent demonstrate improvement in visual functions, but 15 percent of patients benefit very little or not at all. Prolonged mental stress and personality seem to play a key role in preventing vision restoration (Figure 1).”25
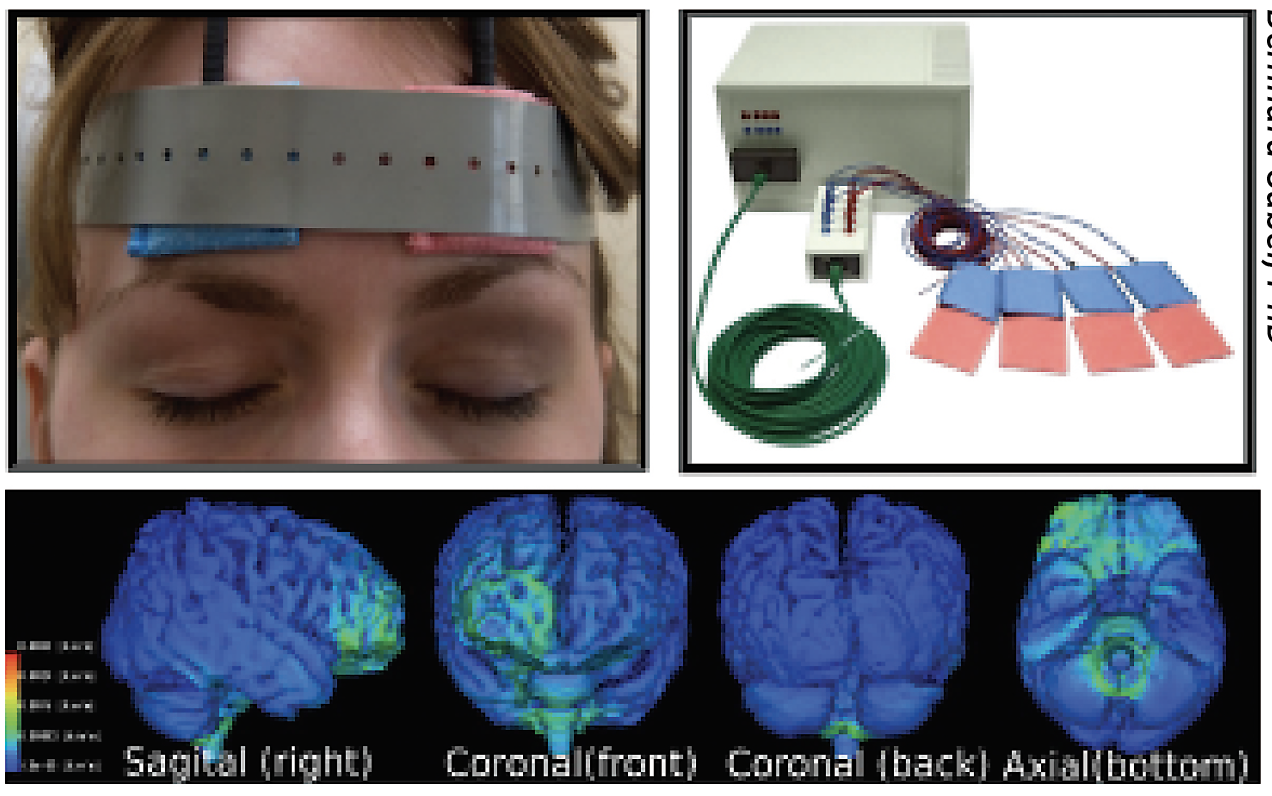 |
| Figure 2. A patient using the at-home Active SAVIR Alpha Synch mobile device for microcurrent stimulation. Current flow in the brain is shown below. (Courtesy Bernhard Sabel, PhD) |
A clinical trial at Stanford University, NYU Langone and Otto-von-Guericke University Magdeburg (NCT05626491) is currently enrolling to test the safety and efficacy of long-term rtACS therapy with an at-home device for open-angle glaucoma and optic neuropathies. The randomized, double-masked study has an estimated enrollment of 45 participants with an estimated completion date of December 2024. The experimental arm includes treatment with the Active SAVIR Alpha Synch mobile device, a headband that delivers electrical stimulation, every other day for eight weeks (Figure 2). The sham arm involves the same device but no active stimulation. The primary outcome is change from baseline in visual field assessed using the Humphrey Visual Field Index through six months. Secondary outcome measures include change from baseline in mean deviation, Pelli-Robson contrast sensitivity and Snellen visual acuity.
A concurrent open-label study (NCT05626426) at Stanford for participants who didn’t fit the exact enrollment criteria for the randomized trial is also recruiting, with an estimated enrollment of 20 participants. This study will also assess RNFL and GCC OCT changes, retinal metabolism, adaptive optics retinal imaging, laser speckle flowgraphy and OCT angiography changes.
Dr. Khouri points out, “The challenges with nerve stimulation studies are that sample sizes are small, and inclusion and exclusion criteria are often restrictive, targeting either patients with very advanced disease or those with very early glaucoma on the disease spectrum. The jury is still out but there’s a lot of interest in neurorestorative or neuroprotective therapies. It remains to be seen whether an intervention will materialize from the research that could help patients, but I think we’re many years away from that.”
Lifestyle Modifications
The level of evidence for lifestyle modifications and their effects on glaucoma is low compared with pharmacological agents, but some modifications may be safely incorporated by patients such as exercise, smoking cessation, weight loss and altered sleeping positions.
“Strenuous physical exercise has been shown to both lower high IOP and increase optic nerve health,” says Dr. Schuman. “Sleeping on 30-degree wedge pillow has been shown to reduce nocturnal elevations in eye pressure in some patients.”
A prospective, nonrandomized comparative case series of 17 eyes of 17 patients with glaucoma, controlled IOP and new disc hemorrhage demonstrated that sleeping in a 30-degree head-up position lowers IOP compared with a flat position, with variable effects between patients (mean IOP was 3.2 mmHg lower, p=0.03).25 All but one patient had lower IOP using the head-up position. IOP reduction was ≥20 percent in 35 percent of patients. No differences in blood pressure or ocular perfusion pressure were found between the two head positions. A subsequent study of head elevation vs. supine position during sleep in 71 open-angle glaucoma patients reported similar results but noted that “resting on multiple pillows” doesn’t seem to reduce IOP uniformly in glaucoma patients.26
“A question simply inquiring about face-down sleep can be relevant,” Dr. Khouri notes. “If patients sleep face down, they’re putting pressure on their eye, and that could have a deleterious effect on their glaucoma. The literature is favorable for exercise, smoking cessation and a healthy body mass index. All of these things can improve oxygen saturation and blood flow. The health of the vascular system matters when it comes to glaucoma.”
One review study reported that exercise had a small effect on glaucoma (1 to 2 mmHg IOP decrease), and that IOP increases due to stress, high wind instrument playing and yoga inversions.27 The authors mentioned it was reasonable to inform glaucoma patients about transient IOP elevations associated with certain activities.
The Bottom Line
“This topic is an important reminder that patients will often self-treat their glaucoma based on inaccurate information and that they may not disclose this to you unless specifically addressed,” Dr. Marando says. “Physicians also have a responsibility to educate patients and to protect them from ineffective and potentially dangerous treatments, which can also be costly and time-consuming.”
Dr. Sabel is the founder of the Savir Center in Magdeburg, Germany. Drs. Schuman, Khouri, Chen and Marando have no related financial disclosures.
1. Weinreb RN, Ong T, Scassellati Sforzolini B, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: The VOYAGER study. Br J Ophthalmol 2015;99:6:738-745.
2. Walters TR, Ahmed IIK, Lewis RA, et al. Once-Daily netarsudil/latanoprost fixed-dose combination for elevated intraocular pressure in the randomized phase 3 MERCURY-2 study. Ophthalmology Glaucoma 2019;2:5:280-289.
3. Santen and UBE receive FDA approval for Omlonti (omidenepag isopropyl ophthalmic solution) 0.002% for the reduction of elevated intraocular pressure in patients with primary open-angle glaucoma or ocular hypertension. Sept 26, 2022. https://www.santen.com/content/dam/santen/global/pdf/en/news/20220926-1.pdf. Accessed April 27, 2023.
4. SPARC Announces Positive Top-line Results from Pivotal Phase 3 Clinical Trial of PDP-716 for the Treatment of Open Angle Glaucoma or Ocular Hypertension. May 14, 2021. https://www.sparc.life/sites/default/files/2021-05/PDP-716%20topline%20results_14th%20May.pdf. Accessed April 27, 2023.
5. Rhee DJ, Katz LJ, Spaeth GL, Myers JS. Complementary and Alternative Medicine for Glaucoma. Survey of Ophthalmology 2001;46:1:43-55.
6. Wan MJ, Daniel S, Kassam F, et al. Survey of complementary and alternative medicine use in glaucoma patients. J Glaucoma 2012;21:2:79-82.
7. Marando CM, Chen TC. Evidence for complementary and alternative therapies to treat glaucoma. Seminars in Ophthalmology 2023;38:1:85-91.
8. Margeta MA. Single Digits but Still Progressing – Now What? Part 1: Neuroprotection. Presented at the 2023 American Glaucoma Meeting in Austin, TX.
9. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits 2015;8(Spec Issue):13-14.
10. Tribble JR, Otmani A, Sun S, et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol 2021;43:101988.
11. Kouassi Nzoughet J, Chao de la Barca JM, Guehlouz K, et al. Nicotinamide deficiency in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2019;60:7:2509-2514.
12. Harder JM, Guymer C, Wood JPM, et al. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or rapamycin. Proc Natl Acad SciUSA 2020;117:52: 33619-33627.
13. De Moraes CG, John SWM, Williams PA, et al. Nicotinamide and pyruvate for neuroenhancement in open-angle glaucoma: A phase 2 randomized clinical trial. JAMA Ophthalmol 2022;140:1:11.
14. Nishimura M, Suzuki M, Takahashi R, et al. Daily ingestion of eggplant powder improves blood pressure and psychological state in stressed individuals: A randomized placebo-controlled study. Nutrients 2019;11:11:2797.
15. Iwamoto K, Birkholz P, Schipper A, et al. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Invest Ophthalmol Vis Sci 2014;55:1078-1087.
16. Yakobashvili D, Shah R, Oydanich M, Khouri AS. Public perception of marijuana use for the treatment of glaucoma. J Glaucoma. Published online March 3, 2023.
17. Skye Bioscience receives human research ethics committee approval to start multiple ascending dose arm of Phase 1 study of SBI-100 ophthalmic emulsion. March 15, 2023. https://ir.skyebioscience.com/news-events/press-releases/detail/160/skye-bioscience-receives-human-research-ethics-committee. Accessed April 27, 2023.
18. Chaudhry S, Dunn H, Carnt N and White A. Nutritional supplementation in the prevention and treatment of glaucoma. Surv Ophthalmol 2022;67:4:1081-1098.
19. Sim R, Sirasanagandla SR, Das S and Teoh SL. Treatment of glaucoma with natural products and their mechanism of action: An update. Nutrients 2022;14:534.
20. Ige M, Liu J. Herbal medicines in glaucoma treatment. Yale J Biology and Med 2020;93:347-353.
21. Gall C, Schmidt S, Schittkowski MP, et al. Alternating current stimulation for vision restoration after optic nerve damage: A randomized clinical trial. PLoS One 2016;11:6:e0156134.
22. Bola M, Gall C, Moewes C, et al. Brain functional connectivity network breakdown and restoration in blindness. Neurology 2014;83:6:542-551.
23. Wu Z, Sabel BA. Spacetime in the brain: Rapid brain network reorganization in visual processing and recovery. Scientific Reports 2021;11:17940.
24. Sabel BA, Flammer J, Merabet LB. Residual vision activation and the brain-eye-vascular triad: Dysregulation, plasticity and restoration in low vision and blindness – A review. RNN 2018;36:6:767-791.
25. Sabel BA, Wang J, Fähse S, et al. Personality and stress influence vision restoration and recovery in glaucoma and optic neuropathy following alternating current stimulation: Implications for personalized neuromodulation and rehabilitation. EPMA Journal 2020;11:2:177-196.
26. Buys YM, Alasbali T, Jin YP, et al. Effect of sleeping in a head-up position on intraocular pressure in patients with glaucoma. Ophthalmology 2010;117:7:1348-1351.
27. Park JH, Nam KT, Yoo C, Kim YY. Head elevation and intraocular pressure in glaucoma. Optom Vis Sci 2016;93:9:1163-1170.
28. Parikh RS, Parikh SR. Alternative therapy in glaucoma management: Is there any role? Indian J Ophthalmol 2011;59 Suppl 1:S158-160.



