Sometimes, in order to improve your outcomes with medical or surgical interventions, you don’t need a new therapy, you just need to apply your current therapy in a new way or—in the case of the suprachoroidal space—in a new place. The suprachoroidal space is a potential space between the sclera and choroid that glaucoma specialists have long taken advantage of for surgical drainage. Today, the SCS has also become a high-value target for retinal therapeutics ranging from intraocular drug and gene delivery to surgical interventions such as scleral buckling and retinal prosthesis implantation. Here, we’ll show you the ways this space is being used, and how it might help improve outcomes.
Anatomy of the SCS
Located between the sclera and the outer border of the choroid, the SCS consists of a gradual transition from the loose lamellar fibers of the choroidal stroma to the more compact fibers of the sclera.1 Under physiologic conditions, the SCS has an average thickness of 35 µm1,2 and is collapsed due to the intraocular pressure.3 In pathologic conditions, serous fluid or blood may collect in the SCS, but won’t extend beyond the scleral spur anteriorly or the optic nerve posteriorly.4 The SCS serves as the intermediary channel for the uveoscleral pathway, by which aqueous flows through the ciliary body into the SCS, through the sclera and out through the lymphatics.5
Imaging the SCS
Although standard OCT penetrates poorly through the retinal pigment epithelium and the light defocuses at the choroid,6 newer advances let us better visualize the choroid and deeper structures. Enhanced depth imaging (EDI)-OCT is obtained by placing an OCT device closer to the eye to create an inverted image with an increased depth of field.7 Similarly, swept source-OCT employs a tunable swept laser with a longer median wavelength of 1,050 nm (as compared to 840 nm in spectral domain-OCT) to allow deeper tissue penetration and better visualization of deeper structures.8
On OCT, the suprachoroidal layer is found at the choroidal-scleral junction and is thought to have an inner hyperreflective band and an outer hyporeflective band.9 The consensus is that the hyperreflective band represents pigmented cells interspersed between fibroblasts while the hyporeflective band represents the SCS.9 The hyporeflective SCS can be seen on EDI-OCT scans of the macula in approximately half of healthy adults above age 50, and its presence correlates with hyperopia.6 The SCS may be more difficult to visualize in those with darker uveal pigment such as those of Asian or African descent.10 The SCS was noted to be visible in 20 percent of those with exudative AMD, and half with non-exudative AMD,9 likely because macular fluid may impair SCS visualization.11
Accessing the SCS
There are several techniques to access the SCS. Minimally-invasive glaucoma surgery traditionally uses the ab interno approach, in which drainage devices such as the now-defunct Cypass Micro Stent (Alcon) and the investigational iStent Supra (Glaukos) are surgically implanted to allow aqueous to flow from the anterior chamber to the SCS without a filtering bleb.12
An external approach can also be achieved by transscleral cannulation (iTrack, iScience Interventional).13 This is performed by creating a full-thickness pars plana scleral flap and threading a microcatheter through the SCS to the intended location. Care must be taken to identify the choroidal-scleral junction to avoid dissecting through the choroid or retina. The catheter has a flashing diode that helps the surgeon visualize the advancing catheter through the surgical microscope.13 The advantage of this technique is that it allows for precise delivery of the drug or substance, though there is a steep learning curve and potential risk for choroidal hemorrhage.
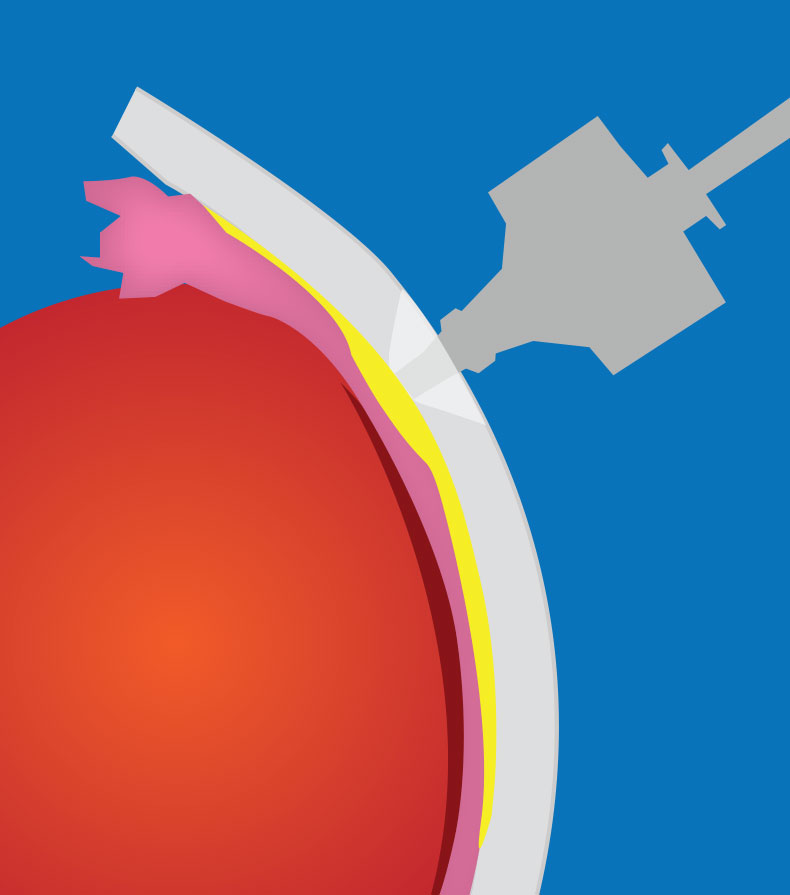 |
| Figure 1. A transscleral microneedle can be used to access the suprachoroidal space. |
Microneedles have been developed to allow easy and safe access to the SCS in the outpatient setting. The microneedles have a depth set to that of the sclera and conjunctiva so that the choroid and retina aren’t inadvertently perforated.14 A hollow-bore 750 um-long microneedle (Clearside Biomedical) is inserted perpendicular to the sclera at the pars plana and often held for ~1 minute to prevent reflux (See Figure 1).13,14 This particular technique is necessary because, unlike standard intravitreal injections, suprachoroidal microneedle injections should be given slowly to minimize patient discomfort.
Pharmacokinetics
Intravitreal injections are the most common route for treating retinal conditions, but efficacy may be limited by biodistribution, tissue penetration and pharmacokinetics. Drug distribution in the vitreous can be non-uniform, as small molecules rapidly distribute through it, while larger molecules are more restricted.15 The internal limiting membrane may also serve as a barrier, for example, in viral-mediated gene therapy.15 Materials in the vitreous are cleared anteriorly through trabecular outflow and posteriorly through passive and active transport through the blood-retina barrier and uveoscleral outflow. Therefore, hydrophilic and larger molecules tend to be cleared more slowly.15
According to a paper from Clearside’s founder and his co-workers, delivery to the SCS bypasses the ILM, spares anterior segment structures unwanted exposure, and provides higher drug concentrations to the retina, RPE and choroid.14 For example, suprachoroidal fluorescein localizes to the choroid and retina at 25- to 200-times-higher levels than with intravitreal injection.16 Similarly, bevacizumab injected into the SCS showed an eightfold higher concentration in the choroid compared to injections into the vitreous.17 Studies in dog and pig eyes have shown that the SCS can accommodate up to 1 mL of fluid as compared to 10 to 50 µL in the vitreous.18 Although there is a circumferential distribution of fluid with SCS delivery, it doesn’t cover the entire space.14 Distribution is limited anteriorly by the scleral spur and posteriorly by the optic nerve. Because the SCS is distensible, a higher injection volume can possibly cover a larger area.14
The three proposed routes of SCS clearance include: 1) from the injection site; 2) pressure-driven, transscleral movement; and 3) diffusion through the choroid and intravascular route.19 Most molecules are cleared more quickly from the SCS than from the vitreous, although lipophilic molecules such as triamcinolone acetonide form precipitates that dissolve slowly. Suprachoroidal injection of triamcinolone took 120 days to clear as compared to 41 days after intravitreal injections in non-vitrectomized eyes and six days in vitrectomized eyes.20 In contrast, bevacizumab was undetectable in the SCS seven days after suprachoroidal injection.17 Molecules up to 500kDa are cleared from the eye two days after SCS delivery, but very large molecules (2MDa) take up to 20 days to be eliminated.19 Interestingly, polystyrene microparticles (20kDa) may last four months or longer in the SCS,21-23 possibly because these rigid microspheres can’t enter the choroidal circulation easily.14 These various properties can be taken advantage of to increase the half-life of various therapeutics in the SCS.
Drug Delivery in the SCS
The use of microneedles to deliver triamcinolone to the SCS has been examined in macular edema associated with noninfectious uveitis (NIU), retinal vein occlusions and diabetes.
• Clearside studies. In Clearside’s Phase III PEACHTREE study, 160 patients with macular edema due to NIU were randomized to a suprachoroidally-injected triamcinolone acetonide suspension (CLS-TA) or sham treatment at weeks 0 and 12.24 Of those receiving CLS-TA, 47 percent gained 15 or more ETDRS letters as compared to 16 percent in the sham arm (p<0.001) at week 24.24 Mean reduction in central subfield thickness (CST) was 153 µm versus 18 µm, respectively (p<0.001).24 The incidence of elevated intraocular pressure and cataract progression were similar between the two arms.
For eyes with RVO-related macular edema, the Phase II TANZANITE study evaluated suprachoroidal CLS-TA combined with intravitreal aflibercept (Eylea, Regeneron) versus intravitreal aflibercept alone.25 Both arms demonstrated comparable visual acuity, but the need for retreatment was significantly lower in the combination arm compared with the aflibercept-only arm (78 vs. 30 percent; p=0.003), suggesting that CLS-TA could reduce injection burden in these patients.25 The Phase I/II HULK trial also evaluated CLS-TA monotherapy or combination treatment with intravitreal aflibercept in patients with diabetic macular edema.26 The treatment was well-tolerated with few adverse events, though VA gains and CST reduction were greater among treatment-naïve eyes as compared to previously-treated eyes.26
Post hoc imaging analyses offer insight into the pharmacokinetics of suprachoroidal drug delivery. While anterior segment OCT of eyes from the HULK study showed SCS expansion immediately post-injection and anatomic restoration at one month,27 macular EDI-OCT of eyes from the TANZANITE study showed slight but persistent SCS expansion after three months, providing some evidence for sustained drug effect.28
Suprachoroidal delivery has also been explored for anti-VEGF pharmacotherapies. Axitinib (Inlyta) is a tyrosine kinase inhibitor that directly blocks VEGF receptors-1, -2, and -3 and is currently approved to treat renal cell cancer.29,30 In a study from Clearside, a single suprachoroidal injection of axitinib in rabbit eyes achieved a higher concentration in posterior segment structures and more sustained VEGF inhibition than standard anti-VEGF-A therapies.30
Initial data from Clearside’s Phase I/IIa OASIS trial (NCT04626128) evaluating 0.03 mg suprachoroidal axitinib suspensions (CLS-AX) for patients with previously treated neovascular AMD showed VA and CST improvements, no adverse events and a reduction in intravitreal injection burden over three months in the first cohort (n=6).29 The study will proceed with dose escalation in cohorts 2 and 3 (0.1 mg and 0.3 mg, respectively).
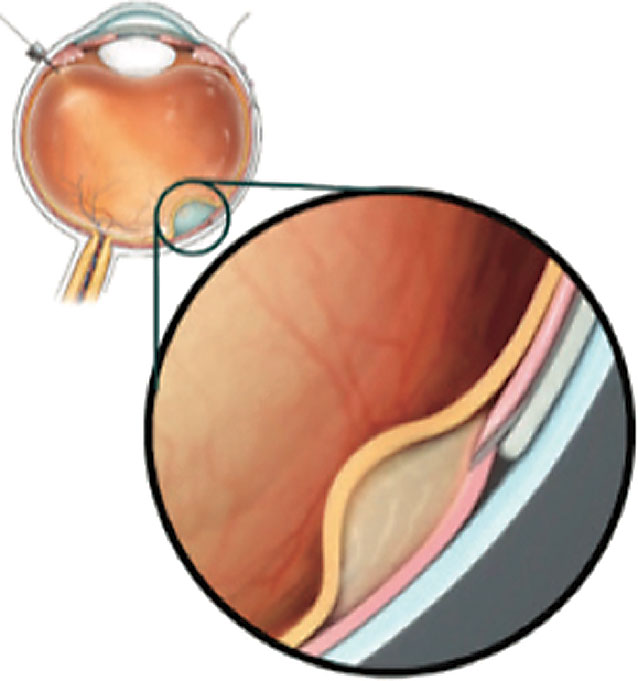 |
| Figure 2. A cannula can be passed through the suprachoroidal space and microneedle into the subretinal space. (Copyright Gyroscope Therapeutics, 2021. Reproduced with permission.) |
• Gyroscope Therapeutics’ studies. The SCS also provides a conduit for drug delivery into the subretinal space. The Orbit Subretinal Delivery System (Orbit SDS, Gyroscope Therapeutics) delivers a precise volume of drug into the subretinal space by passing a cannula through the SCS and advancing a microneedle into the space (See Figure 2).31 As complement dysregulation is a key driver of AMD pathology, the Orbit SDS is being evaluated for an investigational gene therapy, GT005, to increase expression of complement factor I (CFI), which is a negative regulator in the complement alternative pathway.32 Subretinal injection of GT005 has been found to be safe in mouse and nonhuman primate models.32 The Phase I/II FOCUS (NCT03846193), Phase II EXPLORE (NCT04437368) and Phase II HORIZON (NCT04566445) studies are under way to evaluate its safety and efficacy in AMD patients with geographic atrophy.31
Gene Delivery to the SCS
Subretinal injection of viral vectors is currently the preferred method for viral-mediated gene therapy. However, subretinal injection requires surgery in the operating room, and the therapeutic effect is limited to the area of the subretinal bleb. Suprachoroidal injection provides a potentially easier route of delivery while enabling a broader area of gene transduction.
• Delivery of AAV vectors. Suprachoroidal delivery of an AAV8 vector expressing green fluorescent protein (GFP) using a conventional needle demonstrated widespread expression on retinal flat mount tissues across rats, pigs and nonhuman primates.33 Using a similar technique, injection of RGX-314, an AAV8 vector that expresses an anti-VEGF Fab fragment, showed expression levels similar to subretinal injections in rats.33 Our laboratory compared intravitreal, subretinal and suprachoroidal delivery of AAV8 in nonhuman primates using transscleral microneedles, and similarly found widespread GFP expression. However, the transgene expression was mostly limited to the peripheral RPE that was highest at month one; it declined by month three after injection along with infiltration of inflammatory cells.34
In follow-up studies, we found that while intravitreal AAV8-GFP produced low retinal expression and high egress of viral particles to systemic circulation to trigger a neutralizing antibody response to the viral capsid, suprachoroidal delivery of the same vector generated high levels of local GFP expression in the sclera, which is outside the blood-retinal barrier, and thus elicited stronger immune responses to the GFP transgene.34,35
Since GFP is a fluorescent jellyfish protein that’s foreign to the human body, the SCS remains a compelling route for gene therapies with normal human or humanized transgenes. The Phase II AAVIATE (NCT04514653) and ALTITUDE (NCT04567550) trials to evaluate suprachoroidal microneedle delivery of RGX-314 (RegenXBio), which consists of an AAV8 vector that encodes an anti-VEGF antibody fragment, are currently enrolling patients with neovascular AMD (AAVIATE) and center-involving diabetic macular edema (ALTITUDE).36,37
• Nanoparticle delivery. Suprachoroidal delivery of nanoparticles enables non-viral-based gene therapy that may avoid immunogenicity and achieve higher therapeutic doses.38 Subretinal and suprachoroidal delivery of luciferase DNA nanoparticles in rabbits successfully transfects the RPE, choroid and retina.39,40 Suprachoroidal injections of nanoparticles containing a DNA plasmid enabled transgene expression in rat photoreceptors and RPE for at least eight months, and can be used to produce an anti-VEGF protein to suppress subretinal neovascularization.41
Suprachoroidal delivery of viral nanoparticle conjugates has also been evaluated for the treatment of choroidal melanoma. AU-011 (Aura Biosciences) binds to cancer cells through modified heparan sulphate proteoglycans and induces cell death when photoactivated with a non-thermal infrared laser.42
Suprachoroidal injection of AU-011 results in tumor regression in rabbits,42 and is undergoing Phase II investigation in patients with choroidal melanoma.43
Vitreoretinal Surgery in the SCS
The suprachoroidal space can also be used in various surgical applications.
• Retinal implants. The approval of the Argus II retinal implant (Second Sight Medical Products), which uses electrodes to stimulate inner retinal neurons, bypass degenerated photoreceptors and restore basic vision to those with severe vision loss,44 has generated significant enthusiasm for the development of retinal prostheses. These prosthetics may be implanted in epiretinal, subretinal and intrascleral locations to help patients regain some light and object perception,44 though different complications are associated with the various locations and surgical techniques.
Implantation into the SCS doesn’t require manipulation of the retinal tissue and may avoid disrupting the fragile neurosensory retina of patients with retinal degenerations. The first human clinical trial (n=3) using a suprachoroidal retinal prosthetic in RP patients (Bionic Vision Australia Research Consortium) showed improved light localization in all three participants, but was complicated by subretinal and suprachoroidal hemorrhage that formed three to four days after surgery.44 In two patients, the hemorrhage resolved completely, while one patient formed a fibrovascular scar at the temporal edge of the device, though this didn’t affect its efficacy.44 The researchers noted that a longer habituation time may be required to properly assess visual acuity. Following the success of this trial, the team tested a 44-channel suprachoroidal prosthesis in cats that showed a good safety profile.45
• Retinal detachment repair. In addition to retinal prosthesis implantation, the suprachoroidal space may also be accessed for retinal detachment repair. Injection of viscoelastic into the SCS using a microcatheter introduced through a sclerotomy and advanced to the target area enables suprachoroidal buckling to relieve traction from peripheral retinal breaks in rhegmatogenous retinal detachments. It can also be injected underneath the fovea to repair myopic foveoschisis or macular holes.46 Although this eliminates the need for and complications associated with silicone bands in conventional scleral buckling surgery, the steep learning curve and risk for choroidal hemorrhage has limited its widespread adoption.
In conclusion, with new technologies and methods to image and access the SCS, many clinical trials are under way to evaluate novel therapies that involve suprachoroidal drug or gene delivery. Suprachoroidal injections can be performed in outpatient settings using microneedles, and the location and pharmacokinetics of the SCS enable more widespread and targeted delivery of drugs and viral vectors to the outer retina, retinal pigment epithelium and choroid while limiting the impact on anterior segment structures. However, the position of the SCS outside the blood-retinal barrier and juxtaposition to the high-flow choroidal vessels present unique challenges for optimizing drugs’ durability and minimizing exposure to the host’s immune system.
Future innovations to maximize the efficacy and safety of suprachoroidal therapies could further expand the indications for this potentially game-changing route of delivery.
Dr. Mehta is a second-year surgical vitreoretinal fellow at the UC Davis Eye Center. He has no financial interest in any of the products mentioned.
Dr. Yiu is an associate professor of ophthalmology at UC Davis, where he works as a vitreoretinal surgeon and clinician-scientist. His research interests include gene therapy and gene editing, ocular imaging and AMD. He consults for: Alimera; Allergan; Carl Zeiss Meditec; Clearside Biomedical; Genentech; Gyroscope Therapeutics; Intergalactic Therapeutics; Iridex; NGM Biopharmaceutical; Regeneron; Topcon, and also receives research support from Clearside, Genentech and Iridex.
1. Tasman W, Jaeger E. Duane’s Ophthalmology. 15th ed. Philadelphia: Lippincott Williams & Wilkins, 2009.
2. Krohn J, Bertelsen T. Corrosion casts of the suprachoroidal space and uveoscleral drainage routes in the human eye. Acta Ophthalmol Scand 1997;75:32–35.
3. Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci 1989;30:233–238
4. Emami-Naeini P, Yiu G. Medical and Surgical Applications for the Suprachoroidal Space. Int Ophthalmol Clin 2019;59:1:195-207.
5. Alm A, Nilsson SF. Uveoscleral outflow—A review. Exp Eye Res 2009;88:4:760-8.
6. Yiu G, Pecen P, Sarin N, Chiu SJ, Farsiu S, Mruthyunjaya P, Toth CA. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol 2014;132:2:174-81.
7. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008;146:4:496-500.
8. Vira J, Marchese A, Singh RB, Agarwal A. Swept-source optical coherence tomography imaging of the retinochoroid and beyond. Expert Rev Med Devices 2020;17:5:413-426.
9. Michalewska Z, Michalewski J, Nawrocka Z, Dulczewska-Cichecka K, Nawrocki J. Suprachoroidal layer and suprachoroidal space delineating the outer margin of the choroid in swept-source optical coherence tomography. Retina 2015;35:2:244-9.
10. Yiu G, Vuong V, Oltjen S, et al. Effect of uveal melanocytes on choroidal morphology in rhesus macaques and humans on enhanced-depth imaging optical coherence tomography. Invest Ophthalmol Vis Sc 2016;57:13:5764–5771.
11. Wong SS, Vuong VS, Cunefare D, Farsiu S, Moshiri A, Yiu G. Macular fluid reduces reproducibility of choroidal thickness measurements on enhanced depth optical coherence tomography. Am J Ophthalmol 2017;184:108-114.
12. Bailey A, Sarkisian S, Vold S. Ab interno approach to the suprachoroidal space. J Cataract Refract Surg 2014;40:8:1291–1294.
13. Wang J, Eliott D. Accessing the suprachoroidal space for therapeutic delivery. Int Ophthalmol Clin 2017;57:4:179–192.
14. Chiang B, Jung J, Prausnitz M. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev 2018;Epub.
15. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J 2010;12:3:348-360.
16. Tyagi P, Barros M, Stansbury JW, Kompella UB. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Mol Pharm 2013;10:8:2858-67.
17. Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci 2011;52:7:4749-56.
18. Seiler G, Salmon J, Mantuo R, Feingold S, Dayton P, Gilger B. Effect and distribution of contrast medium after injection into the anterior suprachoroidal space in ex vivo eyes. Invest Ophthalmol Vis Sci 2011;52:8:5730–5736.
19. Chiang B, Wang K, Ethier CR, Prausnitz MR. Clearance kinetics and clearance routes of molecules from the suprachoroidal space after microneedle injection. Invest Ophthalmol Vis Sci. 2017;58:1:545-554.
20. Olsen T, Ferg X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol 2006;142:5:777–787.
21. Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res 2011;28:166–176.
22. Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci 2012;53:4433–4441.
23. Park SH, Lee KJ, Lee J, Yoon JH, Jo DH, Kim JH, Kang K, Ryu W. Microneedle-based minimally-invasive measurement of puncture resistance and fracture toughness of sclera. Acta Biomater 2016;44:286-94.
24. Yeh S, Khurana RN, Shah M, Henry CR, Wang RC, Kissner JM, Ciulla TA, Noronha G; PEACHTREE Study Investigators. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: Phase 3 randomized trial. Ophthalmology 2020;127:7:948-955.
25. Campochiaro PA, Wykoff CC, Brown DM, Boyer DS, Barakat M, Taraborelli D, Noronha G; Tanzanite Study Group. Suprachoroidal triamcinolone acetonide for retinal vein occlusion: Results of the Tanzanite Study. Ophthalmol Retina 2018;2:4:320-328.
26. Wykoff CC, Khurana RN, Lampen SIR, Noronha G, Brown DM, Ou WC, Sadda SR; HULK Study Group. Suprachoroidal triamcinolone acetonide for diabetic macular edema: The HULK Trial. Ophthalmol Retina 2018;2:8:874-877.
27. Lampen SIR, Khurana RN, Noronha G, Brown DM, Wykoff CC. Suprachoroidal space alterations following delivery of triamcinolone acetonide: Post-hoc analysis of the Phase 1/2 HULK study of patients with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2018;49:9:692-697.
28. Willoughby AS, Vuong VS, Cunefare D, Farsiu S, Noronha G, Danis RP, Yiu G. Choroidal changes after suprachoroidal injection of triamcinolone acetonide in eyes with macular edema secondary to retinal vein occlusion. Am J Ophthalmol 2018;186:144-151.
29. Clearside Biomedical announces positive safety results from cohort 1 of OASIS Phase 1/2a Clinical Trial of CLS-AX (axitinib injectable suspension) for the treatment of wet AMD. Available online: https://www.globenewswire.com/news-release/2021/06/15/2247133/0/en/Clearside-Biomedical-Announces-Positive-Safety-Results-from-Cohort-1-of-OASIS-Phase-1-2a-Clinical-Trial-of-CLS-AX-axitinib-injectable-suspension-for-the-Treatment-of-Wet-AMD.html
30. Kansara VS, Muya LW, Ciulla TA. Evaluation of long-lasting potential of suprachoroidal axitinib suspension via ocular and systemic disposition in rabbits. Transl Vis Sci Technol 2021;10:7:19.
31. Gyroscope Therapeutics announces first patient received investigational gene therapy GT005 via orbit subretinal delivery system in ongoing Phase I/II FOCUS Trial. Available online: https://www.biospace.com/article/releases/gyroscope-therapeutics-announces-first-patient-received-investigational-gene-therapy-gt005-via-orbit-subretinal-delivery-system-in-ongoing-phase-i-ii-focus-trial/
32. Ellis S, Buchberger A, Holder J, Orhan E, Hughes J. GT005, a gene therapy for the treatment of dry age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci 2020;61:7:2295.
33. Ding K, Shen J, Hafiz Z, et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J Clin Invest 2019;13;129:11:4901-4911.
34. Yiu G, Chung SH, Mollhoff IN, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther Methods Clin Dev 2020;16:179-191.
35. Chung SH, Mollhoff IN, Mishra A, et al. Host immune responses after suprachoroidal delivery of AAV8 in nonhuman primate eyes. Hum Gene Ther 2021 Apr 8. [Epub ahead of print].
36. Regenxbio announces dosing of first patient in Phase II AAVIATE trial of RGX-314 for the treatment of wet AMD using suprachoroidal delivery. Available online: http://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-dosing-first-patient-phase-ii-aaviatetm
37. Regenxbio announces dosing of first patient in Phase II ALTITUDE trial of RGX-314 for the treatment of diabetic retinopathy using suprachoroidal delivery. Available online: http://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-dosing-first-patient-phase-ii-altitudetm
38. Wan CR, Muya L, Kansara V, Ciulla TA. Suprachoroidal delivery of small molecules, nanoparticles, gene and cell therapies for ocular diseases. Pharmaceutics 2021;13:2:288.
39. Kansara V, Yoo J, Cooper MJ, et al. Suprachoroidally delivered non-viral DNA nanoparticles transfect chorioretinal cells in non-human primates and rabbits. Invest Ophthalmol Vis Sci 2019:60:2909.
40. Kansara VS, Cooper M, Sesenoglu-Laird O, et al. Suprachoroidally delivered DNA nanoparticles transfect retina and retinal pigment epithelium/choroid in rabbits. Transl Vis Sci Technol 2020;9:21.
41. Shen J, Kim J, Tzeng SY, et al. Suprachoroidal gene transfer with nonviral nanoparticles. Sci Adv 2020;6:27:eaba1606. Published 2020 Jul 3. [Epub ahead of print].
42. Savinainen A, Grossniklaus H, Kang S, et al. Ocular distribution and efficacy after suprachoroidal injection of AU-011 for treatment of ocular melanoma. Invest Ophthalmol Vis Sci 2020;61:7:3615.
43. Aura Biosciences announces dosing of first patient in Phase 2 study evaluating suprachoroidal administration of AU-011 in patient with choroidal melanoma. Available online: http://www.aurabiosciences.com/news-archive/2020/6/12/aura-biosciences-presents-updated-au-011-clinical-data-at-arvo-2020-njm82
44. Ayton LN, Blamey PJ, Guymer RH, et al. First-in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One 2014;9:12:e115239. Published 2014 Dec 18.
45. Abbott CJ, Nayagam DAX, Luu CD, et al. Safety studies for a 44-channel suprachoroidal retinal prosthesis: A chronic passive study. Invest Ophthalmol Vis Sci 2018;59:3:1410-1424.
46. El Rayes EN, Elborgy E. Suprachoroidal buckling: Technique and indications. J Ophthalmic Vis Res;8:4:393-9.




