Today, 20/20 refractive surgery outcomes have become commonplace. Nevertheless, refractive surgeons continue to look for ways to improve their results, increasing the percentage of patients achieving 20/20—and better.
One of the technologies helping to make these increasingly excellent outcomes possible is topography-guided ablation. Here, surgeons with extensive experience using this technology—as well as refractive surgery systems that integrate topographic information in other ways—share their experience treating both virgin and so-called “20-unhappy” eyes.
The Argument for Topography
Ronald R. Krueger, MD, director of the Truhlsen Eye Institute and McGaw Professor and chairman of the department of ophthalmology at the University of Nebraska Medical Center in Omaha, who uses the WaveLight Allegretto Wave Eye-Q excimer laser platform to perform topography-guided ablations, notes that topography may not intuitively seem like the best data on which to base an ablation. “You’d think that using wavefront data would be the best way to get a great outcome, because you want to treat the optics of the entire eye,” he says. “However, most of the refractive surface is on the cornea, and most of the aberrations are there as well. Furthermore, the resolution of a topography scan is much greater than what you get with a wavefront reading. Topography can give you something like 20,000 points of information about the corneal surface, as compared to 100 or 200 points of optical information from a wavefront reading.
“Topography-guided ablation isn’t a perfect approach, because an eye can have lenticular astigmatism or posterior corneal astigmatism,” he admits. “But the data suggests that topography-guided ablation is helping us give our patients better-quality vision. For example, in one study I published last year involving 256 eyes, the data showed that at three months, uncorrected distance visual acuity was 20/20 or better in 95.7 percent of the eyes and 20/15 or better in 81.4 percent, while 25.6 percent gained one or more lines of corrected distance visual acuity.1 That last number indicates that we corrected some corneal aberrations, since patients gained lines of CDVA.
“I’m currently working on a retrospective study comparing physicians who were treated with wavefront-optimized technology to those treated with topography-guided ablation,” he adds. “The data is showing statistically greater 20/10 and 20/15 results in the topography-guided group. I think this is why topography-guided is gaining some traction; it’s that next increment of creating better vision.”
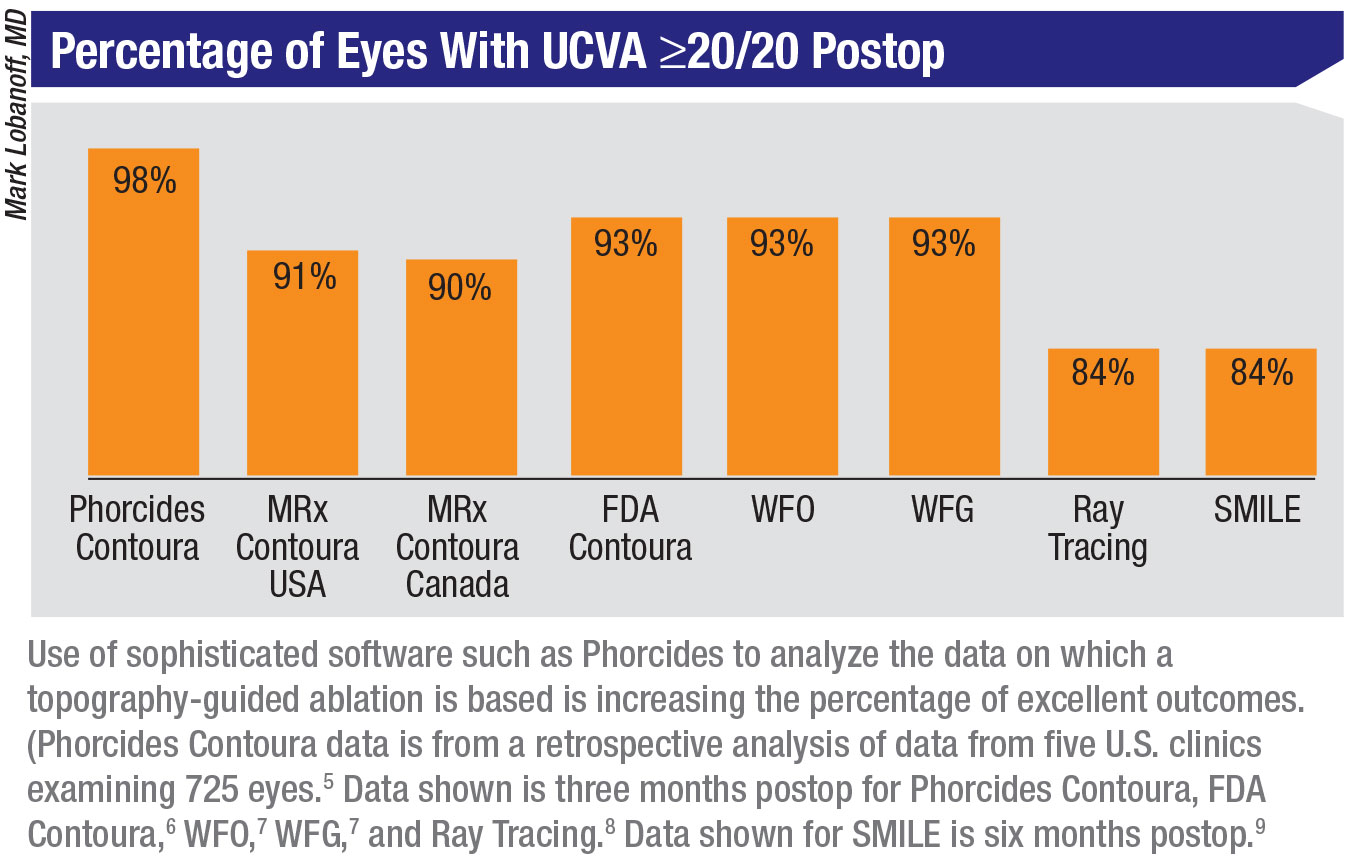 |
Nancy Tanchel, MD, in private practice at Liberty Laser Eye Center in Vienna, Virginia, performs topography-guided ablations using the Nidek Advanced Vision Excimer Laser System (NAVEX) EC-5000 Quest, featuring Customized Aspheric Treatment Zone software (CATz). She explains that the Nidek system can do multiple types of corneal ablation, including topography-guided ablation for myopia and astigmatism. “[The topography-guided ablation] doesn’t completely remove corneal irregularities, however,” she notes. “Instead, it treats about 40 percent of the measured topographic irregularities.
“We use the OPD Scan instrument to measure the eye,” she continues. “It captures both wavefront and topography data, which allows it to separate the internal aberrations from the surface aberrations. The topography-guided ablation then optimizes the light entering the eye using wavefront optimization, while correcting any topographic surface aberrations. In contrast to wavefront-guided ablations, CATz doesn’t try to compensate for internal or lenticular aberrations on the cornea.”
Dr. Tanchel says she’s noted slightly better results using the topography-guided approach. “Patients have fewer night-vision issues,” she says. “The original FDA study found that topography-guided treatment with the Nidek system caused a 23-percent improvement in subjective night-driving symptoms, and we definitely hear fewer complaints about that. As far as improving best-corrected visual acuity, most of our patients are fairly young and have excellent best-corrected visual acuity before the treatment, so it’s hard to assess how much better it is afterwards.”
Taking It To the Next Level
“Topography-guided customized ablation has been around for a long time,” says Karl Stonecipher, MD, medical director for TLC Laser Eye Centers in Greensboro, North Carolina, and clinical associate professor of ophthalmology at the University of North Carolina. (Dr. Stonecipher uses the WaveLight Allegretto Wave Eye-Q excimer laser platform to perform topography-guided ablation.) He notes that topography-guided ablation in the United States has evolved through three formats to date. “At first we used a protocol designed for the FDA trial,” he says. “We put the manifest refraction and the topography into the software and proceeded with the treatment. The biggest problem with the resulting data was that we chose patients for the trial who had ‘normal’ topographies. If the topography looked unusual, that patient wasn’t included in the study. So we tested the software on the crème de la crème of normal corneas.
“Then, John Kanellopoulos and Manoj Motwani pursued the idea of using the amount of astigmatism and axis measured by the Vario Topographer, instead of using the astigmatism data from the manifest refraction,” he continues. “They’ve reported very good outcomes with that approach. However, we’ve tried it and couldn’t duplicate their results; we ended up with a lot of flipped axes. We got better results using the FDA trial protocol.2,3
“The third version of topography-guided ablation has resulted from the advent of the Phorcides software,” he says. “Phorcides is a new software package developed by Mark Lobanoff, MD. Mark looked at a lot of data from his practice and designed software to improve the diagnostics and the way that data is then used to ablate the cornea.
“Phorcides does weight-averaging of several datasets: the standard topographic data, the topography from the WaveLight Vario device, the topography from the Pentacam—for both the anterior and posterior corneal surfaces—and the manifest refraction,” Dr. Stonecipher explains. “The result is a vectored analysis. In particular, Dr. Lobanoff figured out a way to analyze all of the factors relating to astigmatism, whether it’s regular astigmatism, irregular astigmatism or astigmatism caused by a higher-order aberration.”
Dr. Krueger agrees that predicting the outcome is more difficult when astigmatism is an issue—especially if the manifest refraction and your topographic measurements report conflicting data about the amount and/or axis of the astigmatism. “For instance, if the manifest refraction shows a different astigmatism value than you get with topography, you’re kind of at an impasse,” he says. “Which should you treat? By and large, studies suggest that using the topography-based astigmatism will probably produce a better outcome than using the manifest data, but you have to look at each case carefully and understand it very well.
“That’s where some of the latest software can help,” Dr. Krueger notes. “The Phorcides analytical software package does vector analysis on all of the different components of astigmatism and gives you a calculated value to put into the instrument. That’s probably been the most robust solution for getting the best outcome when there’s some disparity in the measurements. In fact, it’s been licensed by Alcon to use as a planning tool to help surgeons come up with the right numbers.”
Fine-tuning Phorcides
Dr. Stonecipher says that a group of surgeons with topography-guided experience decided to see how the Phorcides software did with patients whose measurements showed, for example, completely different axes on topography vs. manifest refraction. “We retrospectively looked at the data from more than 100 cases fitting that description, eyes that were treated with the different topography-guided options,” he explains. “We found that the best outcomes resulted when Phorcides was used. These findings are currently submitted for publication.
“Right now we have more than 600 eyes involved in a prospective contralateral eye study comparing manifest TCAT to Phorcides,” he says. “So far, both groups are doing well. [Dr. Stonecipher showed some preliminary data from this study at the annual meetings of the American Society of Cataract and Refractive Surgery and the European Society for Cataract and Refractive Surgery in 2019.] Next, we want to analyze how these patients did at three months, and find out which patients—if any—didn’t do well with this approach. We’re also putting our data into the cloud, and using ‘deep learning’ to improve the software even further.”
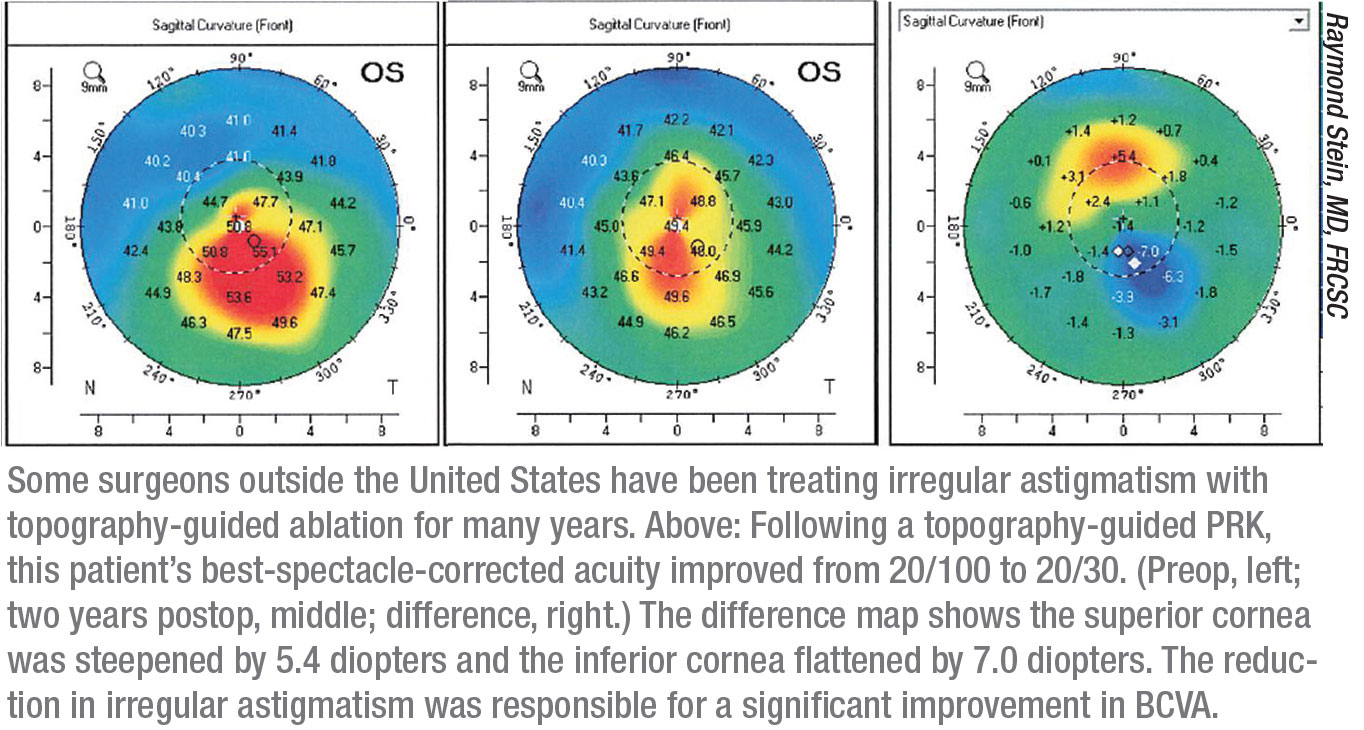 |
Dr. Stonecipher notes that, so far, Phorcides is not designed to analyze eyes with keratoconus or post-LASIK ectasia. “We’re looking at those cases, but it’s currently designed to make a virgin eye see better,” he says. “Nevertheless, the current version of Phorcides is pretty robust. It’s especially useful when dealing with eyes that produce conflicting measurements. To date, I’ve haven’t had to enhance a single eye treated using this software.
“Right now, Dr. Lobanoff is trying to release Phorcides to everyone as a beta software,” Dr. Stonecipher adds. “We’re hoping to improve the outcomes with data from multiple surgeons on multiple platforms at multiple sites.” (Dr. Lobanoff is currently building a module of Phorcides for complex/repair cases, such as off-centered ablations, small ablation zones, post-RK or keratoconus, that will also incorporate epithelial thickness maps into the analytics. That version should be available later this year.)
Treating Irregular Corneas
It’s no secret that topography-guided ablation can be an effective way to address vision problems caused by corneal disease or previous refractive surgeries. Although the FDA approval didn’t include this use, it did make the technology available in the United States. “The FDA tested this technology on primary eyes simply because it was too hard to measure the success of the results with 20-unhappy eyes, and the primary enrollment is reflected in the labeling,” Dr. Krueger says. “But the technology is now accessible for American surgeons to use on these more complex eyes on an off-label basis. If we can fix patients who have an aberrated cornea because of a past correction, it will help reduce any fear of having laser vision correction.”
Meanwhile, some surgeons outside the United States, not having to worry about FDA approval, have accumulated years of experience using this technology to correct more seriously aberrated corneas. One of those surgeons is Raymond Stein, MD, FRCSC, medical director of the Bochner Eye Institute in Toronto and an associate professor of ophthalmology at the University of Toronto. Dr. Stein has been involved with topography-guided treatments for the past 10 years.
“Probably the number one indication we treat using this technology is irregular corneas, such as those with keratoconus, pellucid marginal degeneration or ectasia following laser vision correction,” he says. “Those constitute about 90 percent of the patients we treat. Other less-common indications include patients having post-refractive-surgery complications such as a decentered ablation. That’s pretty rare today, thanks to the use of active tracking systems. The other problem we sometimes treat is a small optical zone, usually the result of surgery done a long time ago. That can cause significant haloes at night when the pupil dilates.”
Dr. Stein says that topography-guided technology allows him to improve best corrected visual acuity. “That’s a big advance,” he says. “We can take an irregular cornea and flatten the steep areas and steepen the flat areas, thus making the cornea more regular and improving best corrected spectacle acuity. We generally use this technology when patients have a best-corrected spectacle acuity of at least 20/30 or worse.”
Taking It Step-by-Step
Dr. Stein describes the steps involved in a typical treatment. “First, we take about eight topographic maps,” he says. “The data is fed digitally into our Allegretto excimer laser, which we’ve used for a number of years. The computer averages the data; then the surgeon selects the depth of the ablation and the optical zone. The larger the optical zone, the more tissue the ablation will remove, and we want to minimize the depth of the ablation. My preference is to use a 6- or 6.5-mm optical zone, because I feel it’s more stable; there’s less chance of regression. However, if the patient has a very thin cornea or a significant difference between the steep and flat areas, we have to use a smaller 5.5- or 5-mm optical zone.”
Dr. Stein notes that removing the epithelium before measuring the topography helps to ensure the best result. “Normally, when we measure corneal topography it’s done with the epithelium intact,” he says. “Many eyes with ectatic disease have a very irregular cornea, and the epithelial thickness can vary from one area to another. For example, in keratoconus, the epithelium tends to be thinner over the cone and thicker out in the periphery. Normally, surgeons rely on preoperative measurements made when the epithelium is intact. However, we’ve found that if we remove the epithelium and then do a topography measurement, the data is significantly different.
“For that reason, we do a phototherapeutic keratectomy first, followed by the topography-guided photorefractive keratectomy,” he explains. “I think you can get reasonable outcomes taking the epithelium off manually, but the results are more accurate if you do a PTK. We remove 50 µm of tissue, usually at a 6.5-mm optical zone with a transition zone at the edge, and then perform the topography-guided PRK. Sometimes, if the cornea is thick enough, we can try to reduce some cylinder at the same time. But overall, we try to limit the total treatment after the PTK to another 50 µm.”
Dr. Stein says that when treating ectatic corneas he applies mitomycin-C for 30 to 60 seconds, irrigates the surface and then proceeds with corneal cross-linking to lock the changes in place. “When treating ectatic disease, we’re weakening the cornea, and there’s a risk of further ectasia,” he says. “That’s why we need to combine this procedure with cross-linking. Also, using mitomycin-C is critical. I’ve been doing PRK for 28 years, and in the early days we had a very high incidence of corneal haze—especially when we did high-diopter treatments. The risk of corneal haze increases slightly when you combine topography-guided PRK with cross-linking; we’ve found that mitomycin-C decreases the incidence of corneal haze.”
 |
Knowing the Limits
Dr. Stein says that a critical factor in the success of these treatments is how much difference there is between the steepest and flattest parts of the cornea. “The results are much better when patients have less than a 10-D difference across the cornea,” he explains. “Those are the eyes in which we get a significant improvement in best-corrected visual acuity. So, within the 6-mm optical zone we look at the steepest part and the flattest part. If the difference is large, such as 20 D, it will be very difficult to smooth that cornea with a topography-guided PRK. We’d have to flatten the steep area by 10 D and steepen the flat area by 10 D, which would be impossible to do. On the other hand, if the difference is less than 10 D, we only need to flatten one area by about 5 D, and steepen another area by 5 D. In fact, our research has revealed that the patients that do the best are those with less than 5 D of difference across the cornea. In those cases, we only have to flatten the steep area by 2.5 D and steepen the flat area by 2.5 D. It’s very easy to smooth those corneas.”
Dr. Stein says that once you’re familiar with the technology, doing the treatment is relatively straightforward. “The most important thing is identifying the patients that are the best candidates,” he notes. “For example, I wouldn’t go out of my way to use this technology to correct irregular astigmatism in a patient with 20/20 acuity. Certainly it’s critical to rule out false ectasia cases, whether it be epithelial basement membrane dystrophy, superficial punctate keratopathy, Salzmann’s nodular degeneration or amiodarone keratopathy. And a map that’s not created properly can produce an irregular topographic pattern where there really isn’t one. You have to be conscientious about looking for those factors so you can rule out poor candidates.
“Beyond those concerns, you should limit the ablation to a certain number of microns,” he continues. “Most of the patients we treat have a corneal thickness of 430 µm or more, because we want to limit our ablation to about 50 µm and have enough thickness left to do cross-linking. We also prefer to treat eyes in which we can create a large optical zone. And, as noted earlier, we have to consider the dioptric difference across the cornea. If the maximum difference is large, we won’t be able to smooth the cornea. The ones
with a difference less than 10 D—
especially less than 5 D—are the best patients to treat with this technology.”
Making the Transition
Dr. Stein notes that the learning curve for using this technology on irregular corneas can be steep. “It takes a number of years to get really comfortable with this,” he says. “It’s a matter of reading as much as you can about this type of procedure, and maybe observing some cases done by surgeons who do this on a regular basis. Of course, right now there aren’t that many surgeons fitting that description. John Kanellopoulos in Greece, David Lin in Vancouver and myself are the ones doing the largest number of irregular cornea treatments.”
Dr. Stein says that he does sometimes treat normal corneas with this technology. “There are some advantages to using this technology, even in that situation,” he notes. “For example, the treatment is centered over the line of sight instead of the center of the pupil. That can improve the quality of vision the treatment produces, especially in hyperopic eyes where the line of sight can be more nasal. Those patients often have a positive angle kappa.”
Dr. Krueger notes that Alcon understands that surgeons hope to eventually use this technology to help those “20-unhappy” patients. “When Alcon released this technology, they said, ‘This isn’t approved for that purpose, so don’t begin by treating those highly aberrated eyes with this technology—you don’t understand it well enough. Do a large number of normal eyes first. Then, when you’re really confortable with the technology, maybe you can selectively use it on previously treated eyes.’
“That’s what I did,” Dr. Krueger says. “It’s helped me to offer some visual improvement to those patients, and I’ve had some successes that were rather dramatic. Sometimes you get patients who have very specific symptoms such as double vision, and you may be able to resolve that by minimizing the irregularities. Sometimes you may have a disillusioned patient who’s lost hope, and you’re giving that person a whole new lease on life.”
Topography-integrated Ablation
Several refractive surgery systems have found other ways to incorporate topographic data into their ablation planning, without using it as the primary basis for the ablation. One such system is the iDesign Refractive Studio (Johnson & Johnson Vision). Marc Odrich, MD, associate professor of ophthalmology at the University of Virginia, has consulted with Johnson & Johnson Vision on the development of the iDesign Refractive Studio. He explains how the current model—version 2.0—incorporates topographic data into its ablation plan.
“The iDesign instruments have always performed a customized treatment,” he notes. “Prior to version 2.0, the system would capture wavefront aberrometry, wavefront refraction, corneal topography, keratometry and pupillometry. The ablation used the wavefront measurement data to create a treatment, also taking into account the keratometry to compensate for the cosine effect. That’s important, because the shape of the cornea affects how the wavefront data propagates from the pupil plane to the corneal plane, and also affects how the laser and cornea interact.
“In the new version of the system, version 2.0, we’re no longer just using keratometry data to adjust how the wavefront correction is delivered,” he explains. “Instead, we’re using the full-gradient topography data the instrument collects. Keratometry provides estimates of the corneal surface details, but only around the central 3 mm; topography provides detailed, specific data about the x and y slopes at 125 points on the corneal surface, allowing the system to compensate more precisely for laser-tissue interactions on that individual cornea.”
Dr. Odrich says the company hasn’t done a head-to-head comparison of the topography-integrated version to previous versions of the system. “However, surgeons using the new version are reporting getting many more 20/10 and 20/12 outcomes than they did before,” he says. “To me, that means the treatments are based on more accurate information. In my own practice I’ve seen the number of 20/10s and 20/12s double. Before, about 40 percent of my patients treated with the system ended up 20/12; now about 80 percent achieve 20/12. That translates into a huge difference in patient satisfaction.”
Dr. Odrich says the 2.0 iDesign Refractive Studio is even easier to use than the previous models, despite incorporating more sophisticated technology. “The acquisition cycle is shorter and more accurate,” he says. “All of the wavefront and topographic data is collected with a single click. The automated exam selection mode uses an algorithm to choose the treatment that’s most appropriate for each individual patient, and the instrument uses iris registration technology to compensate for any eye rotation. I’ve been using this model for seven months now, and I have yet to find a patient whose data I can’t capture, even when eye disease is present.”
 |
Strategies for Success
Surgeons offer the following advice to help you increase the likelihood of excellent outcomes when using topography-guided ablation:
• The quality of the topographic maps is crucial. Dr. Krueger says some patients may not be eligible for topography-guided treatment because they fall outside the approved parameters. “However, that’s fairly rare,” he notes. “A more important issue is the quality of the topographic maps. Garbage in, garbage out. So, make sure there are no dry eyes or other exceptions. If you can’t get a high-quality map because of variability due to dry eye or some other problem, you may want to optimize the cornea as much as possible before proceeding. Or you may say, this is a case where it’s better to just plug in the numbers we have and use wavefront-optimized, rather than trying to base an ablation on data that you can’t be completely certain is accurate.”
Dr. Tanchel agrees, noting that the physician needs to be involved in sorting out which scans are used. “This task shouldn’t be delegated to a lower-level employee in the office,” she says. “You need to look at the topography scans yourself.”
• If the magnitude of astigmatism measured on topography differs from the amount in the manifest refraction, consider treating an amount between those two numbers. Dr. Krueger notes that the magnitude of astigmatism measured on topography is often slightly greater than that measured in the manifest refraction. “At least in the cases in which topography measured a higher value than the manifest refraction, my outcomes suggest it’s best to split the difference,” he says. “Alcon agrees; the company recommends that when you find a disparity between the topographic magnitude of astigmatism and the manifest magnitude, it’s better to treat an amount between the two measurements.”
• If you’re treating irregular corneas, be on the lookout for patients with “false ectasia.” “There are conditions that look like keratoconus or pellucid marginal degeneration,” Dr. Stein explains. “For example, patients with epithelial basement membrane dystrophy may appear to have an irregular cornea on topographic maps, but slit lamp examination will reveal changes within the epithelium. That’s a contraindication for doing a topography-guided treatment. These patients can be helped by just removing the epithelium—essentially, performing a keratectomy.
“There are a number of other conditions like that, such as Salzmann’s nodular degeneration, that can produce an irregular corneal picture,” he continues. “Also, patients on amiodarone for heart problems can have an irregular cornea as a result of drug deposition in the epithelium. These patients should not have a topography-guided treatment. A slit lamp exam, often with fluorescein and a blue light, will allow the clinician to make a diagnosis of these false ectasia cases, which some people call pseudo-keratoconus.” [For more about conditions that mimic ectasia, see Dr. Stein’s recent article in The Canadian Journal of Ophthalmology.4]
• Track your outcomes and note which approaches are producing the best results. Dr. Krueger says this is a key part of using this technology. “I look at the eyes that gained at least one line of best corrected visual acuity after the surgery and analyze how I treated the astigmatism,” he explains. “In those eyes, about 75 percent of the time, I treated the axis of astigmatism measured by the topography, no matter how much the manifest refraction disagreed with it. However, about 25 percent of the time, inputting the manifest axis produced a better outcome. The Phorcides software is now helping to clarify which one should be followed.”
Worth the Effort
Dr. Krueger admits that using this technology takes a little longer than simply plugging a number into your laser and doing a treatment. “You need multiple instruments to gather all the data you require,” he notes. “The Phorcides software is very helpful, but you need a Topolyzer Vario and a Pentacam to use it.
“Nevertheless, in my experience, the payoff is worth it,” he says. “Today I’m getting at least 10 percent more 20/15 outcomes with topography-guided treatments than with wavefront-optimized treatments. Getting more 20/15s and 20/10s is what I want. In a more recent analysis of just my physician patients—because I treated a lot of physicians when I worked at the Cleveland Clinic—I was getting even higher values. Plus, I was getting 100-percent satisfaction rankings from the physicians having topography-guided treatment.”
Dr. Stein notes that no other technology can do what topography-guided ablation can do. “Being able to smooth the cornea and design the treatment specific to the patient’s topography is a significant advance,” he points out. “For any patient with a significant loss of best corrected visual acuity, this is one of the best technologies to use to enhance their vision.”
Dr. Krueger notes that some surgeons believe that topography-guided ablation is just a fad that will eventually pass. “That’s probably true, because something even better will eventually show up,” he says. “In fact we’re about to embark on an FDA study using topography, wavefront and biometry as part of an all-in-one device that collects all of the information. It may eliminate the need for planning software and make the process even easier. But with that in the pipeline, my philosophy is, I want to be as good as I can be right now. I’m offering the technology and its amazing results to patients now, because that’s going to make me more qualified and comfortable with getting into the next level of customization when more advanced versions of this technology hit the market.
“My hope is that eventually we’ll reach a place where the technology is so good that the outcomes are statistically better than glasses or contact lenses in every case. It won’t be just for the person who wants freedom from glasses; it will be for the person who wants to have ‘super vision’—the best he or she can have.
“If our patients consistently gain lines of vision, that’s a game-changer,” he says. “We’ll be improving people’s vision beyond what they could otherwise achieve. Hopefully that will also open up the refractive surgery market in a bigger way.” REVIEW
Dr. Stonecipher has worked with Alcon, VISX, Bausch + Lomb and Nidek. Dr. Krueger is a consultant for Alcon, Johnson & Johnson Vision and Bausch+Lomb Health. Dr. Odrich is a consultant to Johnson & Johnson Vision. Drs. Stein and Tanchel report no relevant financial ties.
1. De Stefano VS, Meister C, Ehlke GL, Krueger RR. Analysis of planning strategies in primary eyes gaining a line or more of visual acuity after topography-guided laser in situ keratomileusis. Jour Cat Refrac Surg 2019;45:3:321-327.
2. Stonecipher, K. Topographic guided ablations: Where are we now and where are we headed? Cataract and Refractive 360. 2018;3:4:1-9.
3. Stonecipher, K, Parrish, J, Stonecipher, M. Comparing wavefront-optimized, wavefront-guided and topography-guided laser vision correction: Clinical outcomes using an objective decision tree. Current Opinion in Ophthalmology 2018;29:4:277–285.
4. Stein R, Salim G. False corneal ectasia in patients referred for corneal cross-linking, topography-guided photorefractive keratectomy, and intrastromal corneal rings. Can Jour Ophthalmolo 2019; 54:3:374-81.
5. Lobanoff M, Mann M, Stonecipher K, Tooma T, Wexler S. Clinical outcomes after topography-guided LASIK calculated with new topography analysis algorithm: Retrospective results from 5 US clinics. Dovepress, In press.
6. FDA Clinical Trials. Allegretto Wave Eye-Q Addendum Procedure Manual T-CAT Topography-Guided Treatments. http://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S012d.pdf
7. Stonecipher K, Kerzirian G. Wavefront-optimized versus wavefront-guided LASIK for myopic astigmatism with the ALLEGRETTO WAVE: Three-month results of a prospective FDA trial. J Refract Surg 2008;24:4:424-30.
8. Schumacher S, Seiler T, Cummings A, Maus M, Mrochen M. Optical ray tracing-guided Laser In Situ Keratomileusis for moderate to high myopic astigmatism. J Refract Surg 2012;38:1:28-34.
9. Sekundo W, Kunert K, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: Results of a 6 month prospective study. Br J Ophthalmol 2011;
95:3:335-9.



