Tuberculosis is a disease caused by airborne transmission and infection with the acid-fast bacillus Mycobacterium tuberculosis.1 Tuberculosis most commonly affects the lungs, but has many extrapulmonary manifestations as well, including intraocular involvement.
While approximately 2 billion people are infected worldwide with M. tuberculosis, only 10 percent of these people will have clinical tuberculosis.2 The prevalence of tuberculosis is highest in Southeast Asia, the western Pacific and Africa, although it is present worldwide. In the United States alone, the Centers for Disease Control and Prevention (CDC) reported 4.9 cases of tuberculosis per 100,000 people in 2004. More than half of these cases were noted in foreign-born residents. The highest rates of tuberculosis in the United States were observed in Asians, African Americans and Latinos.2 HIV infection is a significant risk factor for tuberculosis.3
Among patients with systemic tuberculosis, rates of ocular involvement have varied. A 1967 study of 10,524 patients in a Boston sanatorium, for example, reported a rate of ocular involvement of 1.4 percent.4 In contrast, a study in 1997 of 100 hospitalized patients with tuberculosis in Spain reported a rate of ocular involvement of 18 percent.5 Regardless of rate, intraocular involvement is believed to develop via hematogenous spread from the lung in the vast majority of instances.6 Primary ocular infection in the form of keratitis, conjunctivitis and/or scleritis can occur as well, but is rare.
Ocular Manifestations
Tuberculosis can have a variety of ocular manifestations, and consequently may mimic a number of ocular inflammatory diseases. Moreover, the absence of pulmonary tuberculosis does not rule out the diagnosis of ocular tuberculosis.7 Most commonly, tuberculosis presents as a posterior uveitis.6 A study of 158 clinic patients with intraocular tuberculosis in India over a 10-year period revealed that 42 percent showed posterior uveitis, 36 percent had anterior uveitis, 11 percent had panuveitis and 11 percent had intermediate uveitis.2 Intraocular tuberculosis may also manifest as retinitis, optic neuropathy or endophthalmitis.2
| ||||||||||||
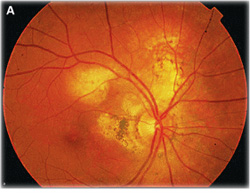
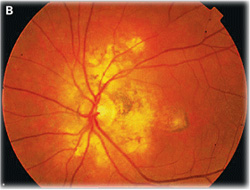
The main types of choroidal involvement in tuberculosis include choroiditis, subretinal abscess, tubercles, and tuberculomas.6 Yellowish subretinal abscesses can occur from liquefaction necrosis within a tubercular granuloma (See Figure 2). Overlying vitritis and retinal hemorrhages are often found associated with the abscess.2 If given time to progress, these abscesses can rupture into the vitreous and result in endophthalmitis.6 However, with appropriate anti-tuberculosis treatment, these abscesses can resolve, leaving a scar.2
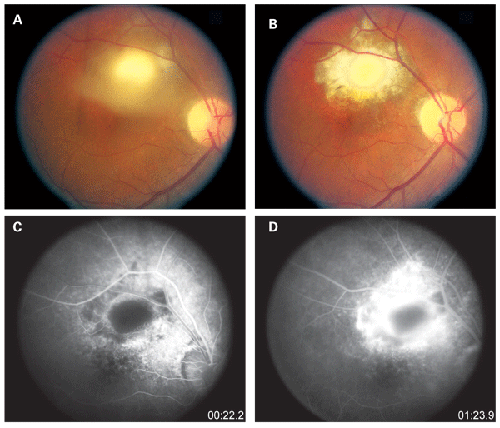
Choroidal tubercules are small gray-white to yellow nodules smaller than a quarter disc diameter, which are not well circumscribed.2,6 Several such nodules can be present at a time, in one or both eyes.2,6 Typically, these tubercles do not have any associated vitritis. When healed, the tubercles appear better circumscribed with surrounding pigment, and develop into a scar.2 A tubercle may grow into a large tumor-like mass up to 14 mm, called a choroidal tuberculoma, which often has a surrounding exudative retinal detachment.6 It is hypothesized that choroidal disease may stem from acid-fast bacilli which subsist in the retinal pigment epithelium.10
Anterior uveitis from tuberculosis often presents with a bilateral iridocyclitis associated with classic, granulomatous, mutton-fat keratic precipitates and posterior synechiae;1,11 tubercular granulomas can also be found within the angle or the iris.1 Occasionally, a robust inflammatory response and purulent material may result in hypopyon formation and possibly endophthalmitis.2 Ocular tuberculosis may also present as an intermediate uveitis in the form of pars planitis. Such findings of tubercular intermediate uveitis include vitritis, vitreous snowballs and snowbanking, peripheral granulomas and vascular sheathing, all possibly complicated by cystoid macular edema.2
The retina can indirectly be involved with ocular tuberculosis due to an associated choroiditis, but direct retinal involvement is rare.2 In the event of primary retinal involvement, the presentation is either as a presumed tubercular retinal vasculitis or as Eales' disease, an associated vasculitis thought to represent a hypersensitivity reaction to tuberculosis.12 Veins are more commonly affected than arteries in tubercular retinal vasculitis, and peripheral capillary occlusion is present.6 Vitritis, retinal hemorrhages and neovascularization are also regularly associated.6 Periphlebitis is often present in Eales' disease as well, but there is no other evidence of intraocular inflammation.2 Additionally, Eales' disease is characterized by peripheral non-perfusion and ischemia, neovascularization, and recurrent vitreous hemorrhage.3
Optic neuropathy can occur from infectious infiltration or inflammation anywhere along the length of the optic nerve, and can present as disc edema or retrobulbar optic neuritis. This optic nerve involvement can occur from hematogenous spread or extension from the choroid.6 Neuroretinitis typically occurs from choroidal spread of infection in a peripapillary location.2
Diagnostic Considerations
Diagnosing ocular tuberculosis can be difficult, and therefore requires many different pieces of evidence. History, clinical appearance, laboratory evaluations, smears and cultures, imaging and pathology are all valuable in making such a diagnosis. Definitive diagnosis of ocular tuberculosis is established by the combination of clinical signs consistent with known patterns of ocular tuberculosis and demonstration of the mycobacterium by means of culture or DNA amplification from ocular samples.
Presumptive diagnosis can be established by the combination of clinical signs consistent with known patterns of ocular tuberculosis with confirmatory ancillary testing, such Mantoux test, interferon-gamma release testing, radiographic findings and nonocular cultures, or a positive response to a four- to six-month therapeutic trial of antituberculous therapy after other possible causes of uveitis have been excluded.2
|
Commercially available interferon-gamma testing is being used as a specific method of detecting tuberculosis, and does not cross-react in patients with prior BCG vaccination or non-tuberculosis mycobacteria.13,14 This in vitro test uses very specific M. tuberculosis antigens to stimulate sensitized T-cells in infected patients and measure the amount of interferon-gamma released from the stimulation.2,13 The chief limitations of this diagnostic strategy are limited availability and increased expense. Nevertheless, these tests are extremely helpful in immunocompromised patients and patients with positive Mantoux testing to rule out false positives from BCG vaccination or non-tuberculosis mycobacteria. Serologic detection of antigens and antibodies associated with tuberculosis has been attempted, but currently is not a widely used clinical method due to low sensitivity and specificity.2,3
A chest X-ray or computed tomography of the chest may reveal active, healed or reactivated tubercular lesions, but can also appear normal.3 The presence of cavities, consolidations or lymph node enlargement is suggestive of primary tuberculosis.15 Primary disease can occur anywhere in the lungs, whereas reactivated disease has a preference for the upper lobes.2 Positive chest X-rays should prompt sputum evaluation.16
Polymerase chain reaction amplification of M. tuberculosis DNA is both a sensitive and specific method for early detection, and is often performed on minute amounts of intraocular fluids and tissue suspected of mycobacterial infection.17,18 These fluids are usually obtained via anterior chamber paracentesis,19 but also occasionally diagnostic vitrectomy or endoretinal biopsy.20
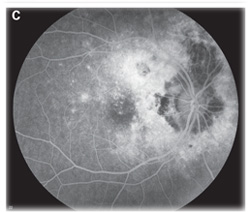
Fluorescein angiography can aid in the diagnosis of ocular tuberculosis. Active choroidal tubercles tend to be hypofluorescent early and hyperfluorescent in later frames.2 Tuberculomas, in contrast, are typically hyperfluorescent early and later pool in the area of associated exudative retinal detachment.2 Choroiditis associated with tuberculosis has a similar fluorescence pattern to serpiginous choroiditis, with early hypofluorescence and later hyperfluorescence or staining of the active border.6 Fluorescein angiography in the setting of retinal vasculitis shows leakage of dye from the vessels, and can also show the presence of peripheral non-perfusion and neovascularization.2 Indocyanine green angiography can also be helpful in diagnosing choroidal lesions. The lesions appear hypofluorescent early, and later have a central zone of hypofluorescence surrounded by hyperfluorescence.6
Due to the sequelae of ocular inflammation in tuberculosis, images from optical coherence tomography are often difficult to obtain and therefore can have limited usefulness in diagnosis. When appropriate images are obtained, OCT may be helpful in detecting uveitic macular edema.21 Ultrasonography may have utility in differentiating tuberculomas from tumors. The mass can be imaged on B-scan and usually has low-to-medium internal reflectivity on A-scan.1,6
Histologically, specimens with tuberculosis classically show granulomatous inflammation with caseous necrosis.15 Necrotic areas may reveal the presence of acid-fast bacilli seen with Ziehl-Neelsen staining.17 Smears with staining and Lowenstein-Jensen media culture of tissue that is suspected of infection may be helpful if such findings are present.6 However, cultures can take up to six to eight weeks because these bacteria are slow-growing.17
In addition to these clinical signs and diagnostic tests, it is important to exclude other diseases that might have a similar appearance to tuberculosis, such as syphilis and toxoplasmosis.2 Response to anti-tuberculosis treatment can also help confirm the diagnosis of tuberculosis22; however, this should be done with care, because such treatment involves committing patients to a lengthy course of antibiotics and their side effects.2
Treatment
Ocular tuberculosis is treated with medical anti-tuberculosis treatment (ATT) similar to that for pulmonary tuberculosis. Current CDC recommendations for active tuberculosis are for four-drug treatment with isoniazid, rifampin, pyrazinamide and ethambutol for two months, followed by an alternate regimen of fewer drugs for four to seven months.23 These alternate regimens may depend on regional resistance patterns, and duration of treatment can be extended in the setting of multi-drug resistance or slow response to treatment.2 Ocular tuberculosis often has a slow response to treatment, and therefore may necessitate longer treatment duration.2 Latent tuberculosis also warrants treatment with isoniazid or rifampin regimens.23 All of these decisions should be made in collaboration with an internist, particularly due to the side effects associated with the medications.
Systemic corticosteroids can be used in conjunction with ATT to prevent damage to ocular tissues from the inflammatory response.2,24 However, corticosteroid treatment alone can cause further progression of the infection, and therefore should not be used without concomitant ATT.2 Other adjuncts to treatment such as rifabutin, fluoroquinolones, interferon-gamma and linezolid are considered in cases of multidrug resistance.2
All active intraocular tuberculosis warrants treatment with ATT. Choroidal tubercles and tuberculomas generally respond well to ATT without any other treatment.6 However, when tuberculosis-associated choroiditis is treated with ATT, initially there may be a paradoxical worsening; concomitant treatment with oral corticosteroids has been considered to circumvent this phenomenon.2 Anterior uveitis associated with tuberculosis is treated with topical cycloplegics and topical corticosteroids, along with ATT.1,11 Oral, depot or topical corticosteroids are also occasionally used in addition to ATT in the treatment of intermediate uveitis in order to prevent cystoid macular edema.2 Management of retinal vasculitis involves both ATT and oral corticosteroids, but also laser photocoagulation of non-perfused retina.1,2
Patients on ATT should be monitored for side effects from treatment. Ethambutol demonstrates dose-dependent toxicity and is rare in patients receiving less than 15 mg/kg.2,3 Optic neuritis, red-green dyschromatopsia, central scotomas, disc edema and optic atrophy are known side effects of ethambutol, and therefore patients should receive baseline eye exams documenting visual acuity, visual field and color vision, and should be followed closely while on treatment.2,3 If any side effects are noted, ethambutol should be immediately discontinued, and vision should return 10 to 15 weeks later.2 Vitamin B12 (hydroxycobalamin) has been used in cases when vision does not return; occasionally, vision loss may be permanent.2 Isoniazid can cause hepatotoxicity and peripheral neuropathy. Rifampin and pyrazinamide are also associated with hepatotoxicity; rifampin is also known to increase the hepatic metabolism of many other drugs.3 Rifabutin can cause a significant anterior uveitis, which responds to topical corticosteroids.1
Intraocular tuberculosis can be difficult to diagnose and treat. A high index of suspicion should be maintained for tuberculosis because of the severity of the disease and its systemic manifestations.
Dr. Shirodkar is a resident in ophthalmology and Dr. Albini an assistant professor of clinical ophthalmology specializing in uveitis and vitreoretinal diseases and surgery at Bascom Palmer Eye Institute. Contact Dr. Albini at BPEI, 900 NW 17th St., Miami, Fla. 33136. Phone: (305) 326-6000; fax: (305) 326-6417; e-mail:
talbini@med.miami.edu. This work was supported by an unrestricted grant from Research to Prevent Blindness, New York City.
1. Tabbara KF. Tuberculosis. Curr Opin Ophthalmol 2007;18(6):493-501.
2. Gupta V, Gupta A, Rao NA. Intraocular tuberculosis—an update. Surv Ophthalmol 2007;52(6):561-87.
3. Bodaghi B, LeHoang P. Ocular tuberculosis. Curr Opin Ophthalmol 2000;11(6):443-8.
4. Donahue HC. Ophthalmologic experience in a tuberculosis sanatorium. Am J Ophthalmol 1967;64(4):742-8.
5. Bouza E, Merino P, Muñoz P, Sanchez-Carrillo C, Yáñez J, Cortés C. Ocular tuberculosis. A prospective study in a general hospital. Medicine (Baltimore) 1997;76(1):53-61.
6. Gupta A, Gupta V. Tubercular posterior uveitis. Int Ophthalmol Clin 2005;45(2):71-88.
7. Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol 2008;146:772-9.
8. Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian J Ophthalmol 2004;52(2):121-5.
9. Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginouslike choroiditis: Clinical presentations and management. Ophthalmology 2003;110:1744-9.
10. Rao NA, Saraswathy S, Smith RE. Tuberculous uveitis: Distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol 2006;124:1777-9.
11. Tabbara KF. Ocular tuberculosis: anterior segment. Int Ophthalmol Clin 2005;45(2):57-69.
12. J.Biswas, T.Sharma, L.Gopal, et al. Eales Disease—An Update. Survey of Ophthalmology 47(3):197-214.
13. Albini TA, Karakousis PC, Rao NA. Interferon-gamma release assays in the diagnosis of tuberculous uveitis. Am J Ophthalmol 2008;146:486-8.
14. Kurup SK, Buggage RR, Clarke GL, Ursea R, Lim WK, Nussenblatt RB. Gamma interferon assay as an alternative to PPD skin testing in selected patients with granulomatous intraocular inflammatory disease. Can J Ophthalmol 2006;41(6):737-40.
15. Helm CJ, Holland GN. Ocular tuberculosis. Surv Ophthalmol 1993;38(3):229-56.
16. Bramante CT, Talbot EA, Rathinam SR, Stevens R, Zegans ME. Diagnosis of ocular tuberculosis: A role for new testing modalities? Int Ophthalmol Clin 2007;47(3):45-62.
17. Arora SK, Gupta V, Gupta A, Bambery P, Kapoor GS, Sehgal S. Diagnostic efficacy of polymerase chain reaction in granulomatous uveitis. Tuber Lung Dis 1999;79(4):229-33.
18. Gupta V, Arora S, Gupta A, Ram J, Bambery P, Sehgal S. Management of presumed intraocular tuberculosis: Possible role of the polymerase chain reaction. Acta Ophthalmol Scand 1998;76(6):679-82.
19. Biswas J, Madhavan HN, Gopal L, Badrinath SS. Intraocular tuberculosis. Clinicopathologic study of five cases. Retina 1995;15:461-8.
20. Cassoux N, Charlotte F, Rao NA, Bodaghi B, Merle-Beral H, Lehoang P. Endoretinal biopsy in establishing the diagnosis of uveitis: A clinicopathologic report of three cases. Ocul Immunol Inflamm 2005;13(1):79-83.
21. Al-Mezaine HS, Al-Muammar A, Kangave D, Abu El-Asrar AM. Clinical and optical coherence tomographic findings and outcome of treatment in patients with presumed tuberculous uveitis. Int Ophthalmol 2008;28(6):413-23.
22. Abrams J, Schlaegel TF Jr. The role of the isoniazid therapeutic test in tuberculous uveitis. Am J Ophthalmol 1982;94:511-5.
23. Centers for Disease Control and Prevention. Treatment of Tuberculosis. MMWR Recomm Rep. 2003 Jun 20;52(RR-11):1-77.
24. Morimura Y, Okada AA, Kawahara S, Miyamoto Y, Kawai S, Hirakata A, Hida T. Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology 2002;109:851-7.