Peripheral retinal ablation has been the mainstay of therapy for
vasoproliferative retinopathy of prematurity dating back to the Cryo-ROP Study. Though highly effective, laser photoablation—the current
treatment standard2—is not a perfect treatment modality. Effective
application of laser is often quite challenging. Timely treatment
requires a thorough familiarity with the nuances of the clinical
features of ROP, particularly posterior disease. Clinicians wellversed
in the ROP diagnosis and laser application are not available
worldwide.Infants afflicted with aggressive ROP may fare poorly despite
timely and appropriate treatment. Though the advantages of peripheral
retinal ablation far outweigh the consequences of untreated ROP, ocular
and systemic morbidities are not so trivial as to be dismissed.
 ROP is a biphasic disease characterized by oxygen-induced attenuation of
normal retinal vascularization followed by hypoxia-induced
vasoproliferation.3 Both phases are mitigated by growth factors,
including the proangiogenic cytokine vascular endothelial growth factor.
A growing body of laboratory and clinical evidence supports the
rationale of targeted pharmacologic inhibition of VEGF as a treatment
for acute phase ROP.4,5
ROP is a biphasic disease characterized by oxygen-induced attenuation of
normal retinal vascularization followed by hypoxia-induced
vasoproliferation.3 Both phases are mitigated by growth factors,
including the proangiogenic cytokine vascular endothelial growth factor.
A growing body of laboratory and clinical evidence supports the
rationale of targeted pharmacologic inhibition of VEGF as a treatment
for acute phase ROP.4,5
What follows is a brief review of the evidence available regarding antiVEGF pharmacotherapy of ROP, either adjunctive to peripheral retinal ablation or as monotherapy.
Literature: Case Reports/Series
The clinical literature speaking to the off-label use of the monoclonal anti-VEGF antibody bevacizumab (Avastin) given by intravitreal injection for infants with advanced ROP consists of case reports/case series and one clinical trial. Both lines of evidence provide clinical information of interest to the management of ROP.
The case series and case report literature on anti-VEGF therapy for ROP is highly variable as to treatment indications, dosage, treatment timing, treatment frequency, outcome measures, follow-up and more. Three main indications for treatment have emerged:
Literature: Clinical Trials
Two prospective randomized trials have been designed to investigate the effi cacy of intravitreal bevacizumab.
• BLOCK-ROP. The BLOCK-ROP study (Pan-VEGF Blockade for the Treatment of Retinopathy of Prematurity, clinical trials identifier NCT00702819) was designed to assess the safety and tolerability of bevacizumab in infants with aggressive posterior ROP (APROP) who had failed conventional laser therapy. Eleven premiere clinical centers with stateof- the-art neonatal intensive care units and clinicians highly experienced in the diagnosis and treatment of ROP were involved in the trial. This phase of BLOCK-ROP was terminated due to insufficient enrollment—ROP progression with timely and effective laser for APROP was rare at BLOCK-ROP study centers: Two infants were enrolled over the course of a year (unpublished data).
A second phase of the BLOCK-ROP study (NCT01232777) targets all treatment- eligible infants (Type-1 threshold ROP),3 with an internal control for more rigorous comparison of treatment efficacy. This dose-ranging Phase II study will aim to demonstrate noninferiority of intravitreal bevacizumab compared with standard-of-care laser.In this randomized three-armed trial, one group of infants will receive 0.75 mg intravitreal bevacizumab in one eye and laser treatment in the fellow eye; a second group will receive 0.625 mg intravitreal bevacizumab in one eye and laser treatment in the fellow eye; and a third group of infants will receive laser photocoagulation in both eyes. It is hoped that, by this study design, the relative risk of increased myopia, amblyopia, and functional outcomes can be assessed, since the eye treated with bevacizumab can be compared with the fellow, laser-treated eye of the same infant. Additionally, a lower effective dose of bevacizumab may be established.
• BEAT-ROP. In the BEAT-ROP study,1 150 infants with bilateral Stage 3+ ROP in zone I or posterior zone II were randomized to receive either intravitreal bevacizumab monotherapy (0. 625 mg in 0.025 cc) or laser photocoagulation in each eye. The primary outcome measure was recurrence of retinal neovascularization between 50 and 70 weeks post-conceptional age, ascertained in 143 surviving infants. In eyes with zone I ROP treated with bevacizumab, recurrence was significantly less than in eyes treated with standard laser photocoagulation (6 percent compared with 42 percent, p=0.003). In eyes with posterior zone II ROP, recurrence was lower in eyes treated with bevacizumab (5 percent Compared with 12 percent, p=0.27), but not to a statistically signifi cant degree. The timing of recurrence was much later in eyes treated with bevacizumab (16 weeks compared with six weeks).
Macular dragging was seen more commonly in laser-treated eyes (16 of 66 eyes with zone I ROP and six of 80 eyes with zone II ROP). Vitrectomy was performed in 13 of 66 eyes treated with laser for zone I ROP and in two of 78 eyes treated with bevacizumab for zone II ROP. Seven infants died (five treated with bevacizumab and two treated with laser). No systemic safety signals were raised, but no systemic adverse events were defined a priori as outcome measures.
Encouraging Data
Case reports/case series have provided some interesting and thoughtprovoking information. They have demonstrated the utility of bevacizumab in eyes with anterior segment features precluding clear visualization of the posterior segment. In such eyes, regression of ROP (either with or without accompanying laser photocoagulation) has been reported to occur in nearly 90 percent of cases.7,11,13-15 Conversely, among previously treated eyes with persistent disease activity despite peripheral retinal ablation, the best response appears to be in eyes with persistent plus disease or exudation without significant fi brovascular proliferation.6,9-14,18
The most encouraging and provocative data come from reports of bevacizumab monotherapy for posterior ROP. Regression, usually with a single injection, has been reported in over 90 percent of cases in this group.8,11,16,19 Most striking is the observation that retinal vascularization may occur, often to the far retinal periphery,6 suggesting that either physiologic VEGF levels recover following acute inhibition, or that retinal vascularization may proceed by VEGF-independent mechanisms. However, complete peripheral retinal vascularization does not occur uniformly (See Figures 3 & 4).
Phase 1 of the BLOCK-ROP study is of interest as it demonstrated that laser peripheral ablation is a highly effective therapy for ROP in this patient population—as this was the reason for non-recruitment.
The BEAT-ROP study provided proof, in the context of a clinical trial, of the prevailing concept that appropriately timed intraocular VEGF inhibition can eliminate most cases of acute ROP. Yet in many respects the BEAT-ROP study data raise more questions than they answer:
The prospect of eradicating a blinding disease with a single injection is an exciting one. It is likely that there will be an important role for anti-VEGF therapy in the management of ROP. However, important unanswered questions remain regarding the role of anti-VEGF therapy for ROP. In contrast to peripheral retinal ablation, for which standard of care parameters have been derived from high-quality prospective multicenter studies, there is no standard approach as to when and how bevacizumab is to be used. Similarly, none of the published literature to date provides adequate guidance as to the intervals or duration of post-treatment follow-up when bevacizumab is the treatment administered.
The conceptualization of ROP as a “window disease” is overly simplistic, as ROP treatment requires more than suppression of the acute phase of disease. Available evidence to date does not yet support broad adoption of anti-VEGF as primary or adjunctive treatment for ROP. For the moment, peripheral retinal ablation remains the treatment of choice. Well-designed clinical trials of anti-VEGF therapy for ROP are needed in order to provide information as to the balance of risk vs. benefit, as well as practical guidance regarding optimal treatment parameters and follow-up.
1. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol 1988;106:471-9.
2. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684- 94.
3. Smith LEH. Through the eyes of a child: Understanding retinopathy through ROP: The Friedenwald lecture. Invest Ophthalmol Vis Sci 2008;49:5177-5182.
4. Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009;116:1599- 603.
5. Sonmez K, Drenser KA, Capone A Jr, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology 2008;115:1065-1070.
6. Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging 2007;38(3):233-7.
7. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 2007;245(11):1727-30.
8. Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008;28:831-8.
9. Kusaka S, Shima C, Wada K, et al. Effi cacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol 2008;92(11):1450-5.
10. Lalwani GA, Berrocal AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008;28(3 Suppl):S13-8.
11. Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, et al. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina 2008;28(3 Suppl):S19-25.
12. Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 2008;246(7):1061-3.
13. Law JC, Recchia FM, Morrison DG, et al. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS 2010;14(1):6-10.
14. Altinsoy HI, Mutlu FM, Güngör R, Sarici SU. Combination of laser photocoagulation and intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging 2010;9:1-5.
15. Lee JY, Chae JB, Yang SJ, et al. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefe’s Arch Clin Exp Ophthalmol 2010 Apr 15 (epub).
16. Dorta P, Kychenthal A. Treatment of type I retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina
2010;30:S24-S31.
17. Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, et al. Paradoxical vascular-fi brotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye 2010;24(5):931- 3.
18. Kychenthal A, Dorta P. Vitrectomy after intravitreal bevacizumab (Avastin) for retinal detachment in retinopathy of prematurity. Retina 2010;30(4 Suppl):S32-6.
19. Wu W, Yeh P, Chen S, et al. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in Taiwan. Ophthalmology 2011 Jan;118(1):176-83. Epub 2010 Jul 29.
20. Bhavsar AR, Googe JM Jr, Stockdale CR, et al. Risk of endophthalmitis after intravitreal drug injection when topical
antibiotics are not required: The diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol 2009;127:1581-3.
21. Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008;28:1395-9.
22. Kong L, Mintz-Hittner HA, Penland RL, Kretzer FL, Chevez Barrios P. Intravitreal bevacizumab as anti-vascular endothelial growth factor therapy for retinopathy of prematurity: A morphologic study. Arch Ophthalmol 2008;126:1161-1163.
23. Martinez-Castellanos MA, Quiroz-Mercado H, Guerrero- Naranjo J, et al. Antiangiogenic therapy in the treatment for retinopathy of prematurity, four years follow up. What we have learned. Invest Ophthalmol Vis Sci 2010;51: E-Abstract 5294.
24. Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: Natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol 2002;120:1470-6.
25. Hoang QV, Kiernan DF, Chau FY, et al. Fluorescein angiography of recurrent retinopathy of prematurity after initial intravitreous bevacizumab treatment. Arch Ophthalmol 2010;128:1080-1.
26. Gilbert WS, Quinn GE, Dobson V, et al. Partial retinal detachment at 3 months after threshold retinopathy of prematurity. Long-term structural and functional outcome. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1996;114:1085-91.
27. Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol 2008;26(3):399-405.
28. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology
2007;114:855-9.
29. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006;113:1695.e1-15.
30. Reddy R, Recchia F, Steele S, Lu P. Systemic adverse events among infants with retinopathy of prematurity (ROP) treated with intravitreal bevacizumab: Determination of a statistically valid cohort. Invest Ophthalmol Vis Sci 2010;51: E-Abstract 5228.
31. Mintz-Hittner HA, Kennedy KA, Chuang AZ, et al. Effi cacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603-15.
32. Drenser KA, Trese MT, Capone A Jr. Aggressive posterior retinopathy of prematurity. Retina 2010;30:Suppl:S37-S40.
33. Capone A Jr, Diaz-Rohena R, Sternberg P Jr, Mandell B, Lambert HM, Lopez PF. Diode-laser photocoagulation for zone 1 threshold retinopathy of prematurity. Am J Ophthalmol 1993;116:444-50.
 ROP is a biphasic disease characterized by oxygen-induced attenuation of
normal retinal vascularization followed by hypoxia-induced
vasoproliferation.3 Both phases are mitigated by growth factors,
including the proangiogenic cytokine vascular endothelial growth factor.
A growing body of laboratory and clinical evidence supports the
rationale of targeted pharmacologic inhibition of VEGF as a treatment
for acute phase ROP.4,5
ROP is a biphasic disease characterized by oxygen-induced attenuation of
normal retinal vascularization followed by hypoxia-induced
vasoproliferation.3 Both phases are mitigated by growth factors,
including the proangiogenic cytokine vascular endothelial growth factor.
A growing body of laboratory and clinical evidence supports the
rationale of targeted pharmacologic inhibition of VEGF as a treatment
for acute phase ROP.4,5
What follows is a brief review of the evidence available regarding antiVEGF pharmacotherapy of ROP, either adjunctive to peripheral retinal ablation or as monotherapy.
Literature: Case Reports/Series
The clinical literature speaking to the off-label use of the monoclonal anti-VEGF antibody bevacizumab (Avastin) given by intravitreal injection for infants with advanced ROP consists of case reports/case series and one clinical trial. Both lines of evidence provide clinical information of interest to the management of ROP.
The case series and case report literature on anti-VEGF therapy for ROP is highly variable as to treatment indications, dosage, treatment timing, treatment frequency, outcome measures, follow-up and more. Three main indications for treatment have emerged:
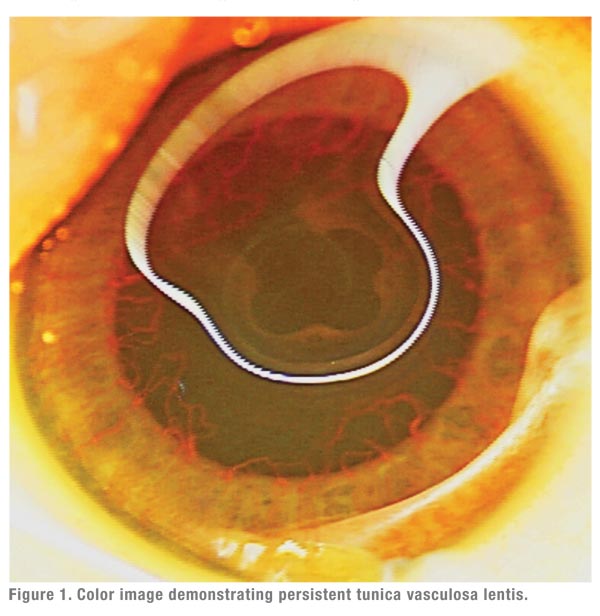
- treatment-naïve eyes with vascular congestion of the anterior segment
precluding adequate visualization for laser treatment (See Figure
1).7,11,13-15
- previously treated eyes with persistent plus disease, exudation, evolving vitreoretinal traction, or tractional retinal detachment.6,9-14,18
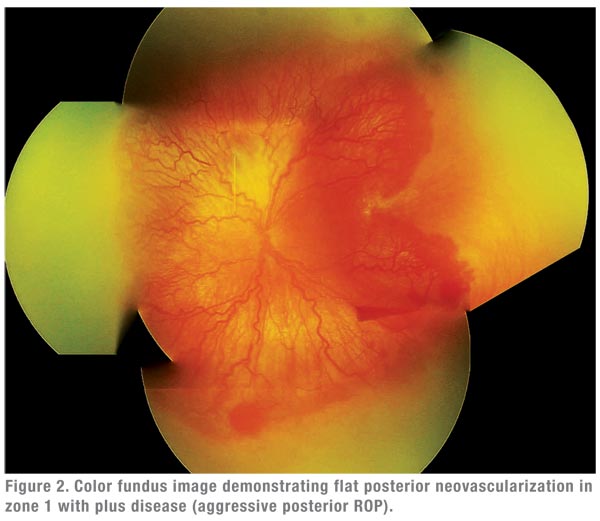
- primary monotherapy (without adjuvant retinal ablation) for eyes with posterior or aggressive disease (See Figure 2).8,11,16,19
Literature: Clinical Trials
Two prospective randomized trials have been designed to investigate the effi cacy of intravitreal bevacizumab.
• BLOCK-ROP. The BLOCK-ROP study (Pan-VEGF Blockade for the Treatment of Retinopathy of Prematurity, clinical trials identifier NCT00702819) was designed to assess the safety and tolerability of bevacizumab in infants with aggressive posterior ROP (APROP) who had failed conventional laser therapy. Eleven premiere clinical centers with stateof- the-art neonatal intensive care units and clinicians highly experienced in the diagnosis and treatment of ROP were involved in the trial. This phase of BLOCK-ROP was terminated due to insufficient enrollment—ROP progression with timely and effective laser for APROP was rare at BLOCK-ROP study centers: Two infants were enrolled over the course of a year (unpublished data).
A second phase of the BLOCK-ROP study (NCT01232777) targets all treatment- eligible infants (Type-1 threshold ROP),3 with an internal control for more rigorous comparison of treatment efficacy. This dose-ranging Phase II study will aim to demonstrate noninferiority of intravitreal bevacizumab compared with standard-of-care laser.In this randomized three-armed trial, one group of infants will receive 0.75 mg intravitreal bevacizumab in one eye and laser treatment in the fellow eye; a second group will receive 0.625 mg intravitreal bevacizumab in one eye and laser treatment in the fellow eye; and a third group of infants will receive laser photocoagulation in both eyes. It is hoped that, by this study design, the relative risk of increased myopia, amblyopia, and functional outcomes can be assessed, since the eye treated with bevacizumab can be compared with the fellow, laser-treated eye of the same infant. Additionally, a lower effective dose of bevacizumab may be established.
• BEAT-ROP. In the BEAT-ROP study,1 150 infants with bilateral Stage 3+ ROP in zone I or posterior zone II were randomized to receive either intravitreal bevacizumab monotherapy (0. 625 mg in 0.025 cc) or laser photocoagulation in each eye. The primary outcome measure was recurrence of retinal neovascularization between 50 and 70 weeks post-conceptional age, ascertained in 143 surviving infants. In eyes with zone I ROP treated with bevacizumab, recurrence was significantly less than in eyes treated with standard laser photocoagulation (6 percent compared with 42 percent, p=0.003). In eyes with posterior zone II ROP, recurrence was lower in eyes treated with bevacizumab (5 percent Compared with 12 percent, p=0.27), but not to a statistically signifi cant degree. The timing of recurrence was much later in eyes treated with bevacizumab (16 weeks compared with six weeks).
Macular dragging was seen more commonly in laser-treated eyes (16 of 66 eyes with zone I ROP and six of 80 eyes with zone II ROP). Vitrectomy was performed in 13 of 66 eyes treated with laser for zone I ROP and in two of 78 eyes treated with bevacizumab for zone II ROP. Seven infants died (five treated with bevacizumab and two treated with laser). No systemic safety signals were raised, but no systemic adverse events were defined a priori as outcome measures.
 |
Case reports/case series have provided some interesting and thoughtprovoking information. They have demonstrated the utility of bevacizumab in eyes with anterior segment features precluding clear visualization of the posterior segment. In such eyes, regression of ROP (either with or without accompanying laser photocoagulation) has been reported to occur in nearly 90 percent of cases.7,11,13-15 Conversely, among previously treated eyes with persistent disease activity despite peripheral retinal ablation, the best response appears to be in eyes with persistent plus disease or exudation without significant fi brovascular proliferation.6,9-14,18
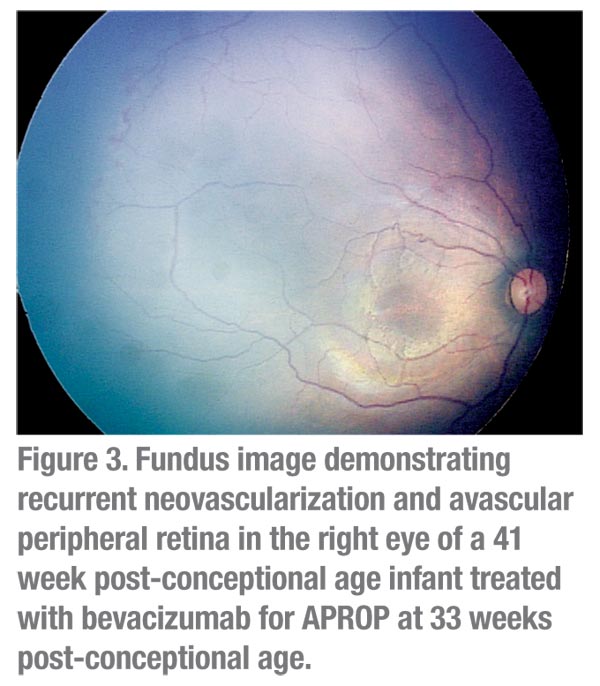
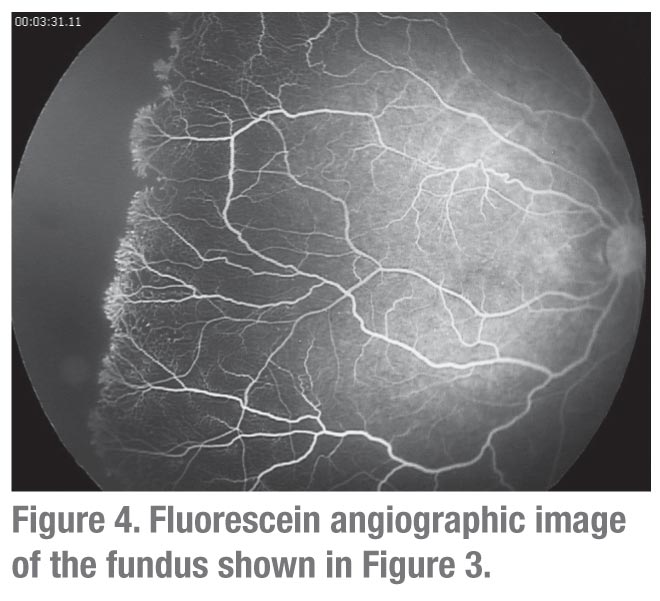
The most encouraging and provocative data come from reports of bevacizumab monotherapy for posterior ROP. Regression, usually with a single injection, has been reported in over 90 percent of cases in this group.8,11,16,19 Most striking is the observation that retinal vascularization may occur, often to the far retinal periphery,6 suggesting that either physiologic VEGF levels recover following acute inhibition, or that retinal vascularization may proceed by VEGF-independent mechanisms. However, complete peripheral retinal vascularization does not occur uniformly (See Figures 3 & 4).
 |
Phase 1 of the BLOCK-ROP study is of interest as it demonstrated that laser peripheral ablation is a highly effective therapy for ROP in this patient population—as this was the reason for non-recruitment.
The BEAT-ROP study provided proof, in the context of a clinical trial, of the prevailing concept that appropriately timed intraocular VEGF inhibition can eliminate most cases of acute ROP. Yet in many respects the BEAT-ROP study data raise more questions than they answer:
- Ten of the 15 BEAT-ROP study centers were located in south and west Texas and more than half of enrolled infants were Hispanic. As a result, the study outcomes may be confounded by population bias, and the results potentially not generalizable.
- The failure rate for ROP following laser in BEAT-ROP is unusually high compared to outcomes in the ETROP study.2 Again, Phase 1 of the BLOCK-ROP study was terminated for precisely the opposite reason—laser failure was a rare event. In a recent study of infants with AP-ROP, laser treatment alone was successful in more than 80 percent of eyes.32 A study of zone 1 ROP from 20 years ago yielded similar results.33 It is reasonable to ask why BEAT-ROP laser outcomes were so inferior to those in the peer-reviewed literature. If similar laser success rates had been achieved in BEAT-ROP, it is less likely that the results would be statistically significant for zone 1 disease.
- The tendency to persistent broad areas of avascular peripheral retina (See Figures 3 & 4) and late recurrence of ROP in eyes treated with bevacizumab alone means that these infants will require extended follow-up. The mean time of recurrence was 16 weeks post-treatment, which is well beyond the time frame within which most infants are discharged. In the real-world, post-NICU setting, follow-up visits to the ophthalmologist may be missed among the myriad other specialist visits. Older infants are more challenging to examine. Given the necessity of these visits, as well as the medicolegal concerns regarding missed visits (the leading underlying cause for litigation), significantly more efforts for patient tracking, follow-up and documentation may be required. There are no data available to provide guidance as to whether eyes with persistent avascular retina are best observed or treated.
The prospect of eradicating a blinding disease with a single injection is an exciting one. It is likely that there will be an important role for anti-VEGF therapy in the management of ROP. However, important unanswered questions remain regarding the role of anti-VEGF therapy for ROP. In contrast to peripheral retinal ablation, for which standard of care parameters have been derived from high-quality prospective multicenter studies, there is no standard approach as to when and how bevacizumab is to be used. Similarly, none of the published literature to date provides adequate guidance as to the intervals or duration of post-treatment follow-up when bevacizumab is the treatment administered.
The conceptualization of ROP as a “window disease” is overly simplistic, as ROP treatment requires more than suppression of the acute phase of disease. Available evidence to date does not yet support broad adoption of anti-VEGF as primary or adjunctive treatment for ROP. For the moment, peripheral retinal ablation remains the treatment of choice. Well-designed clinical trials of anti-VEGF therapy for ROP are needed in order to provide information as to the balance of risk vs. benefit, as well as practical guidance regarding optimal treatment parameters and follow-up.
Dr. Recchia is an associate professor of ophthalmology at Vanderbilt University. Contact him at: Vanderbilt Eye Institute, 2311 Pierce Ave., Nashville, TN 37232-8808. Phone: (615) 936-1457; fax: (615) 936-1540; or e-mail:
franco.recchia@vanderbilt.edu.
Dr. Capone is a clinical professor of biomedical sciences at Oakland University-William Beaumont Hospital School of Medicine in Auburn Hills, MI. He practices at Associated Retinal Consultants, Royal Oak, MI. Contact him at 3535 W. 13 Mile Rd., Suite 344, Royal Oak, MI 48073. Phone: (248) 288-2280; fax: (248) 288-5644; or e-mail: acaponejr@yahoo.com.
Dr. Capone is a clinical professor of biomedical sciences at Oakland University-William Beaumont Hospital School of Medicine in Auburn Hills, MI. He practices at Associated Retinal Consultants, Royal Oak, MI. Contact him at 3535 W. 13 Mile Rd., Suite 344, Royal Oak, MI 48073. Phone: (248) 288-2280; fax: (248) 288-5644; or e-mail: acaponejr@yahoo.com.
1. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol 1988;106:471-9.
2. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684- 94.
3. Smith LEH. Through the eyes of a child: Understanding retinopathy through ROP: The Friedenwald lecture. Invest Ophthalmol Vis Sci 2008;49:5177-5182.
4. Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009;116:1599- 603.
5. Sonmez K, Drenser KA, Capone A Jr, Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology 2008;115:1065-1070.
6. Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging 2007;38(3):233-7.
7. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 2007;245(11):1727-30.
8. Mintz-Hittner HA, Kuffel RR Jr. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008;28:831-8.
9. Kusaka S, Shima C, Wada K, et al. Effi cacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol 2008;92(11):1450-5.
10. Lalwani GA, Berrocal AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008;28(3 Suppl):S13-8.
11. Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, et al. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina 2008;28(3 Suppl):S19-25.
12. Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 2008;246(7):1061-3.
13. Law JC, Recchia FM, Morrison DG, et al. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS 2010;14(1):6-10.
14. Altinsoy HI, Mutlu FM, Güngör R, Sarici SU. Combination of laser photocoagulation and intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging 2010;9:1-5.
15. Lee JY, Chae JB, Yang SJ, et al. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefe’s Arch Clin Exp Ophthalmol 2010 Apr 15 (epub).
16. Dorta P, Kychenthal A. Treatment of type I retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina
2010;30:S24-S31.
17. Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, et al. Paradoxical vascular-fi brotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye 2010;24(5):931- 3.
18. Kychenthal A, Dorta P. Vitrectomy after intravitreal bevacizumab (Avastin) for retinal detachment in retinopathy of prematurity. Retina 2010;30(4 Suppl):S32-6.
19. Wu W, Yeh P, Chen S, et al. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in Taiwan. Ophthalmology 2011 Jan;118(1):176-83. Epub 2010 Jul 29.
20. Bhavsar AR, Googe JM Jr, Stockdale CR, et al. Risk of endophthalmitis after intravitreal drug injection when topical
antibiotics are not required: The diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol 2009;127:1581-3.
21. Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008;28:1395-9.
22. Kong L, Mintz-Hittner HA, Penland RL, Kretzer FL, Chevez Barrios P. Intravitreal bevacizumab as anti-vascular endothelial growth factor therapy for retinopathy of prematurity: A morphologic study. Arch Ophthalmol 2008;126:1161-1163.
23. Martinez-Castellanos MA, Quiroz-Mercado H, Guerrero- Naranjo J, et al. Antiangiogenic therapy in the treatment for retinopathy of prematurity, four years follow up. What we have learned. Invest Ophthalmol Vis Sci 2010;51: E-Abstract 5294.
24. Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: Natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol 2002;120:1470-6.
25. Hoang QV, Kiernan DF, Chau FY, et al. Fluorescein angiography of recurrent retinopathy of prematurity after initial intravitreous bevacizumab treatment. Arch Ophthalmol 2010;128:1080-1.
26. Gilbert WS, Quinn GE, Dobson V, et al. Partial retinal detachment at 3 months after threshold retinopathy of prematurity. Long-term structural and functional outcome. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 1996;114:1085-91.
27. Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol 2008;26(3):399-405.
28. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology
2007;114:855-9.
29. Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006;113:1695.e1-15.
30. Reddy R, Recchia F, Steele S, Lu P. Systemic adverse events among infants with retinopathy of prematurity (ROP) treated with intravitreal bevacizumab: Determination of a statistically valid cohort. Invest Ophthalmol Vis Sci 2010;51: E-Abstract 5228.
31. Mintz-Hittner HA, Kennedy KA, Chuang AZ, et al. Effi cacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603-15.
32. Drenser KA, Trese MT, Capone A Jr. Aggressive posterior retinopathy of prematurity. Retina 2010;30:Suppl:S37-S40.
33. Capone A Jr, Diaz-Rohena R, Sternberg P Jr, Mandell B, Lambert HM, Lopez PF. Diode-laser photocoagulation for zone 1 threshold retinopathy of prematurity. Am J Ophthalmol 1993;116:444-50.



