Presentation and Initial Work-up
Two months prior to presentation, the patient noticed intermittent flashes of light in both eyes. She initially presented to her primary ophthalmologist, who discovered a hypopigmented choroidal lesion in the mid-periphery of the right eye. There was concern for amelanotic choroidal melanoma, and she was referred to the Ocular Oncology Service for further evaluation. Upon presentation to the clinic, she reported intermittent photopsia in both eyes, without floaters. She denied changes in vision, loss of vision, metamorphopsia, pain or other symptoms.
Medical History
Upon initial review, she reported a past medical history of type 2 diabetes mellitus, hypertension, hyperlipidemia, osteoarthritis, kidney stones and vertigo. Her current medications included metformin, sitagliptin, glimepiride, insulin detemir, lisinopril, atorvastatin, aspirin, magnesium, naproxen and meclizine. She denied a personal history of skin cancer or other known malignancy. She reported a surgical history of bilateral cataract extraction two years earlier, right breast mass biopsy (reportedly benign), hysterectomy for fibroids 18 years ago and left oophorectomy for ovarian cyst 16 years ago.
Family history was significant for glaucoma (maternal grandmother, mother, sister), breast cancer (sister, multiple aunts), colon cancer (maternal grandmother, paternal grandfather), and unspecified brain tumor (maternal grandmother). Social history was negative for tobacco, alcohol or other substance use.
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
 |
|
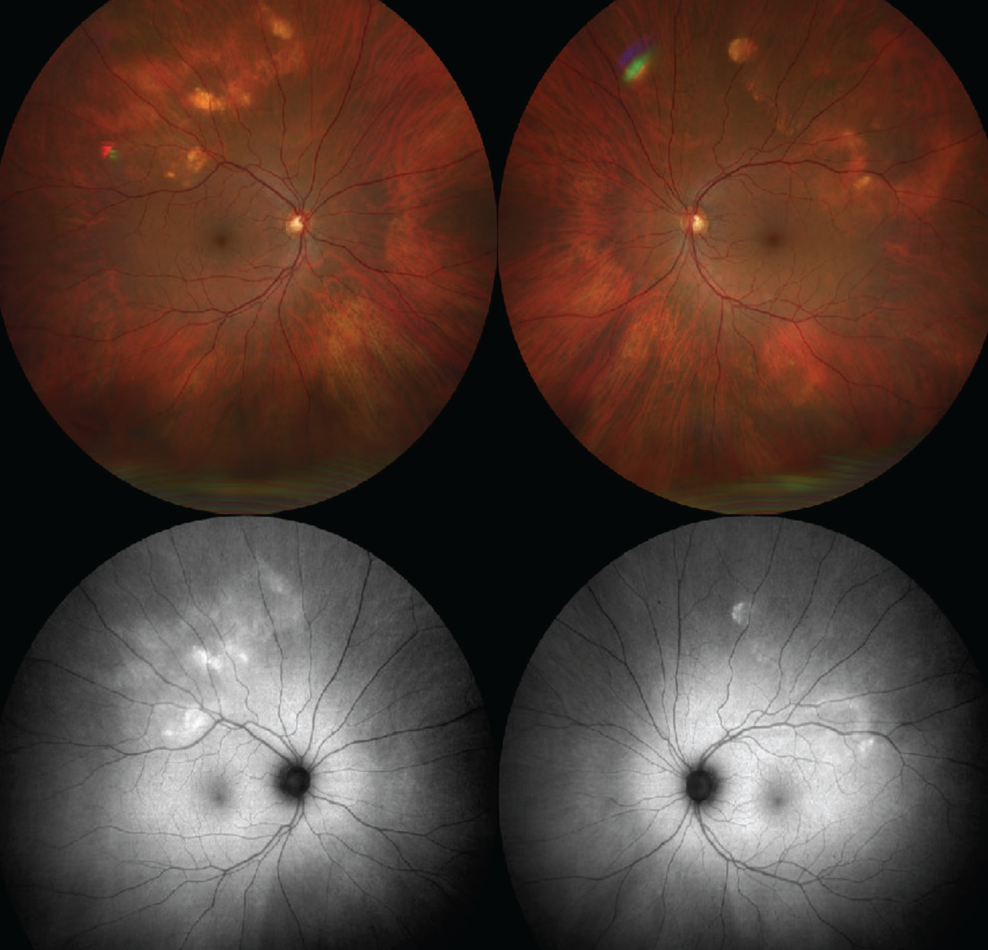
Figure 1. (Top) Color fundus photos demonstrate geographic, yellow/white choroidal mass in the superotemporal mid-periphery of both eyes. (Bottom) FAF shows the lesions to be hyper-autofluorescent. |
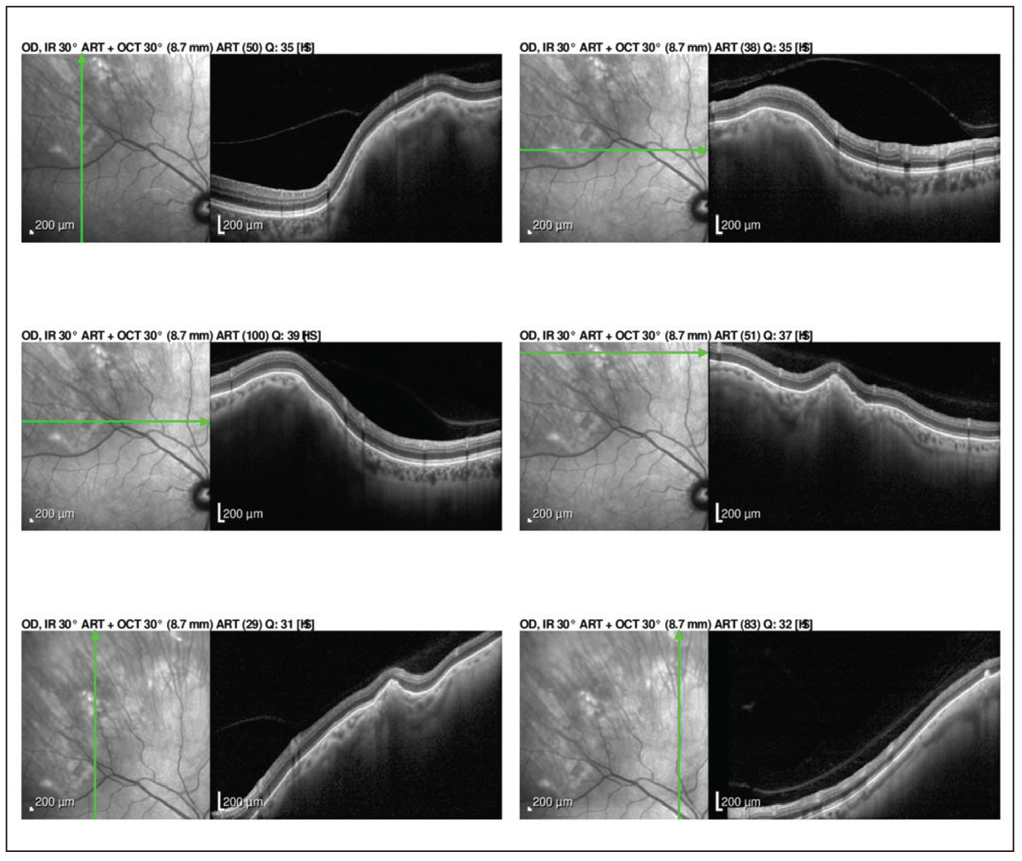 |
| Figure 2. Enhanced depth imaging OCT of the lesions show abrupt rounded and jagged scleral thickening with overlying choroidal and retinal external compression in both eyes. |
Work-up, Diagnosis and Treatment
Ocular examination demonstrated a visual acuity of 20/20 in each eye. Pupils were found to be 3 mm, round and reactive without a relative afferent pupillary defect in either eye. Intraocular pressure was 13 mmHg and 18 mmHg in the right and left eye, respectively. Extraocular motility and confrontation visual fields were full in both eyes. External examination was within normal limits and there was no evidence of melanocytosis or iris heterochromia in either eye. Slit lamp examination revealed posterior chamber intraocular lenses with mild posterior capsular opacification in both eyes.
Dilated fundus examination of the right eye demonstrated a geographic, yellow/white choroidal mass in the superotemporal mid-periphery measuring 10x7 mm (Figure 1). In the left eye, similar lesions were noted in the superotemporal mid-periphery measuring 12x7 mm (Figure 1). Examination was also notable for partial posterior vitreous detachment in the right eye. Otherwise, the vitreous, optic disc, vessels, macula and peripheral retina were within normal limits in both eyes.
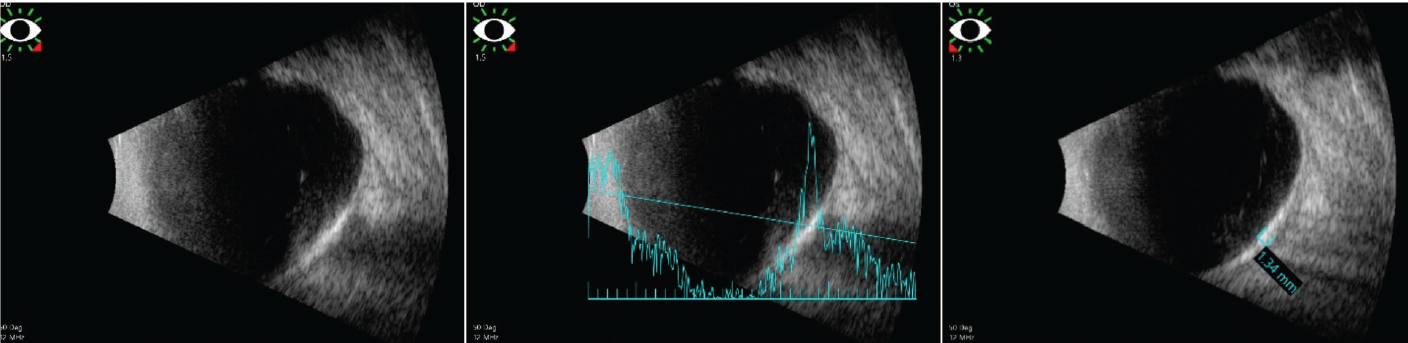
To further evaluate these amelanotic lesions, the patient underwent fundus autofluorescence imaging, optical coherence tomography and B-scan ultrasonography of both eyes. FAF showed hyper-autofluorescence in the distribution of the lesions in both eyes (Figure 1). OCT demonstrated normal retinal thickness and architecture in the macula. However, enhanced-depth OCT imaging of the lesions revealed abrupt, round, jagged scleral thickening with overlying choroidal and retinal external compression in both eyes (Figure 2). B-scan demonstrated the lesions to be placoid and markedly hyperechoic with dense shadowing in both eyes, suggestive of calcification (Figure 3).
Based on these findings, the patient was diagnosed with sclerochoroidal calcification in both eyes.
Upon more targeted questioning, the patient reported that five years prior she was found to have hypercalcemia on routine laboratory evaluation. Subsequent studies showed elevated urine calcium levels and elevated serum parathyroid hormone (PTH). She declined treatment at that time, but two years later was again found to have elevated serum calcium and PTH levels. She was diagnosed with a benign parathyroid adenoma which was surgically removed with resolution of the hypercalcemia.
Discussion
Sclerochoroidal calcification (SCC) is a benign, uncommon condition in which calcium is deposited in the post-equatorial sclera, with secondary compression of the choroid and retina. Given its rarity, the true prevalence of SCC is unclear, but one estimate placed its incidence at around three to six cases per million. The mean age of presentation in one large case series of 118 affected patients was 69 years of age.
Nearly all cases of SCC are asymptomatic from an ophthalmic perspective and discovered on routine fundus examination.1 Rarely, patients present with visual loss or metamorphopsia from retinal complications (discussed below). On dilated fundus exam, SCC typically appears as a yellow/white, flat/placoid lesion(s) at the level of the sclera.2,3 Variable degrees of atrophy in the overlying choroid and retinal pigment epithelium impart a geographic or multifocal appearance.2 The most common location for SCC is the superotemporal quadrant of the midperiphery, or just outside the retinal vascular arcades.2 While it can be unilateral, SCC is frequently bilateral and somewhat asymmetric.1,2
 |
|
Figure 3. B-scan demonstrates the lesions to be placoid and markedly hyperechoic with a large A-scan spike and dense shadowing in both eyes. |
Due to the rarity of this condition, several studies have focused on similar-appearing conditions such as choroidal nevus, choroidal melanoma, choroidal lymphoma, choroidal metastasis, choroidal osteoma and focal choroiditis.2,3 In one reported case, a patient received radiation for suspected prostate cancer metastases that later were determined to be idiopathic SCC.4
The diagnosis of SCC is made with a combination of imaging modalities, most importantly indirect ophthalmoscopy and B-scan ultrasonography. On B-scan, SCC is intensely reflective, with dense shadowing visible even on low gain.1,2,3 OCT, FAF and fluorescein angiography are also frequently obtained. Enhanced depth imaging OCT has been used to categorize SCC into four “mountain-like” types based on their characteristic appearance.5 OCT is also useful for assessing disruption of the overlying choroid, RPE and retinal layers. FAF can be variable, but typically shows hyper-autofluorescence with occasional patches of hypo-autofluorescence. This has been postulated in some cases to represent local disruption of the RPE, either from direct pressure or disruption of underlying choroidal circulation.1 FA can again be variable, but typically shows hyperfluorescence with persistent late staining.1,2,3 FA is perhaps most useful in identifying surrounding CNV.
Once the diagnosis of SCC is made, there is no direct ophthalmic treatment and the patient is observed. In the vast majority of cases, no ill effects arise in the retina or in the patient’s vision.2 However, case reports of choroidal neovascularization associated with SCC support routine monitoring of all identified lesions.6,7
Most importantly, an ophthalmologist should be aware of the systemic associations with SCC, as this diagnosis frequently requires additional medical evaluation. While most reported cases are idiopathic, 21 percent of patients with SCC in one case series were found to have a systemic cause.2 Examples of conditions that can result in metastatic calcium deposition include hyperparathyroidism, elevated vitamin D, renal transport abnormalities, pseudogout, various malignancies and sarcoidosis.8 Of these, the most commonly associated with SCC appear to be parathyroid adenoma, Gitelman syndrome and Bartter syndrome. In the previously mentioned case series, these conditions occurred in 15 percent, 11 percent and 2 percent of patients, respectively. In addition to harm from an undiagnosed underlying condition, hypercalcemia itself is associated with significant morbidity. Kidney stones, polyuria, constipation, nausea/vomiting, pancreatitis, depression, psychosis and CNS disturbance are symptoms of hypercalcemia and primary hyperparathyroidism. This is remembered with the mnemonic “stones, bones, groans, thrones, and psychiatric overtones.”2 Therefore, patients diagnosed with SCC should undergo screening with serum and urine studies including calcium, phosphorus, magnesium, potassium, calcitonin and parathyroid hormone.
In conclusion, sclerochoroidal calcification is an important ophthalmic finding to recognize. Frequently asymptomatic, SCC can be identified by its classic yellow/white geographic appearance, frequent bilateral and superotemporal distribution, and classic appearance on ultrasound, OCT, FAF, and FA imaging. It is important to distinguish SCC from more sinister ocular neoplasia, as SCC typically only needs routine ophthalmic surveillance. Nevertheless, all patients diagnosed with SCC should undergo thorough metabolic evaluation given its unique association with systemic electrolyte derangement. The patient presented above demonstrates a classic example of sclerochoroidal calcification in the setting of hypercalcemia secondary to a parathyroid adenoma. She currently follows with ophthalmology and endocrinology. At the time of this writing, she has shown no evidence of hypercalcemia recurrence, choroidal neovascularization or other ophthalmic complication of SCC.
1. Kivelä TT. Sclerochoroidal Calcification. In: Rojanaporn D. (eds) Ocular Oncology. Retina Atlas. Springer, Singapore, 2019;147-58.
2. Shields CL, Hasanreisoglu M, Saktanasate J, et al. Sclerochoroidal calcification: Clinical features, outcomes, and relationship with hypercalcemia and parathyroid adenoma in 179 eyes. Retina 2015;35:547-54.
3. Sivalingam A, Shields CL, Shields JA, et al. Idiopathic sclerochoroidal calcification. Ophthalmology 1991;98:720-4.
4. Lim JI, Goldberg MF. Idiopathic sclerochoroidal calcification. Arch Ophthalmol 1989;107:1122.
5. Hasanreisoglu M, Saktanasate J, Shields PW, Shields CL. Classification of sclerochoroidal calcification based on enhanced depth imaging optical coherence tomography ‘Mountain-like’ Features. Retina 2015;35:1407–1414.
6. Cohen SY, Guyot-Sionnest G, Puech M. Choroidal neovascularization as a late complication of hyperparathyroidism. American Journal of Ophthalmology 1998;126:320–322.
7. Zaheen M, Sellar W, Mucci B. Idiopathic sclerochoroidal calcification. Eye 2000;14:681–684.
8. Shields JA, Shields CL. “CME review: Sclerochoroidal calcification.” Retina 2002;22:251–261.



